From Wikipedia, the free encyclopedia
Skeletal formula of arginine |
||
|
||
| Names | ||
|---|---|---|
| IUPAC names
Arginine |
||
| Other names
2-Amino-5-guanidinopentanoic acid |
||
| Identifiers | ||
|
CAS Number |
|
|
|
3D model (JSmol) |
|
|
| 3DMet |
|
|
|
Beilstein Reference |
1725411, 1725412 D, 1725413 L | |
| ChEBI |
|
|
| ChEMBL |
|
|
| ChemSpider |
|
|
| DrugBank |
|
|
| ECHA InfoCard | 100.000.738 |
|
| EC Number |
|
|
|
Gmelin Reference |
364938 D | |
|
IUPHAR/BPS |
|
|
| KEGG |
|
|
| MeSH | Arginine | |
|
PubChem CID |
|
|
| RTECS number |
|
|
| UNII |
|
|
|
CompTox Dashboard (EPA) |
|
|
|
InChI
|
||
|
SMILES
|
||
| Properties | ||
|
Chemical formula |
C6H14N4O2 | |
| Molar mass | 174.204 g·mol−1 | |
| Appearance | White crystals | |
| Odor | Odourless | |
| Melting point | 260 °C; 500 °F; 533 K | |
| Boiling point | 368 °C (694 °F; 641 K) | |
|
Solubility in water |
14.87 g/100 mL (20 °C) | |
| Solubility | slightly soluble in ethanol insoluble in ethyl ether |
|
| log P | −1.652 | |
| Acidity (pKa) | 2.18 (carboxyl), 9.09 (amino), 13.8 (guanidino) | |
| Thermochemistry | ||
|
Heat capacity (C) |
232.8 J K−1 mol−1 (at 23.7 °C) | |
|
Std molar |
250.6 J K−1 mol−1 | |
|
Std enthalpy of |
−624.9–−622.3 kJ mol−1 | |
|
Std enthalpy of |
−3.7396–−3.7370 MJ mol−1 | |
| Pharmacology | ||
|
ATC code |
B05XB01 (WHO) S | |
| Hazards | ||
| GHS labelling: | ||
|
Pictograms |

|
|
|
Signal word |
Warning | |
|
Hazard statements |
H319 | |
|
Precautionary statements |
P305+P351+P338 | |
| Lethal dose or concentration (LD, LC): | ||
|
LD50 (median dose) |
5110 mg/kg (rat, oral) | |
| Safety data sheet (SDS) | L-Arginine | |
| Related compounds | ||
|
Related alkanoic acids |
|
|
|
Related compounds |
|
|
| Supplementary data page | ||
| Arginine (data page) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references |
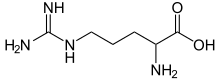
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the l-arginine (symbol Arg or R) enantiomer is found naturally.[1] Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG.[2] The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide.[3] Like all amino acids, it is a white, water-soluble solid.
History[edit]
Arginine was first isolated in 1886 from yellow lupin seedlings by the German chemist Ernst Schulze and his assistant Ernst Steiger.[4][5] He named it from the Greek árgyros (ἄργυρος) meaning «silver» due to the silver-white appearance of arginine nitrate crystals.[6] In 1897, Schulze and Ernst Winterstein (1865–1949) determined the structure of arginine.[7] Schulze and Winterstein synthesized arginine from ornithine and cyanamide in 1899,[8] but some doubts about arginine’s structure lingered[9] until Sørensen’s synthesis of 1910.[10]
Sources[edit]
Production[edit]
It is traditionally obtained by hydrolysis of various cheap sources of protein, such as gelatin.[11] It is obtained commercially by fermentation. In this way, 25-35 g/liter can be produced, using glucose as a carbon source.[12]
Dietary sources[edit]
Arginine is classified as a semiessential or conditionally essential amino acid, depending on the developmental stage and health status of the individual.[13] Preterm infants are unable to synthesize arginine internally, making the amino acid nutritionally essential for them.[14] Most healthy people do not need to supplement with arginine because it is a component of all protein-containing foods[15] and can be synthesized in the body from glutamine via citrulline.[16][17] Additional dietary arginine is necessary for otherwise healthy individuals temporarily under physiological stress, for example during recovery from burns, injury or sepsis,[17] or if either of the major sites of arginine biosynthesis, the small intestine and kidneys, have reduced function, because the small bowel does the first step of the synthesizing process and the kidneys do the second.[3]
Arginine is an essential amino acid for birds, as they do not have a urea cycle.[18] For some carnivores, for example cats, dogs[19] and ferrets, arginine is essential,[3] because after a meal, their highly efficient protein catabolism produces large quantities of ammonia which need to be processed through the urea cycle, and if not enough arginine is present, the resulting ammonia toxicity can be lethal.[20] This is not a problem in practice, because meat contains sufficient arginine to avoid this situation.[20]
Animal sources of arginine include meat, dairy products, and eggs,[21][22] and plant sources include seeds of all types, for example grains, beans, and nuts.[22]
Biosynthesis[edit]
Arginine is synthesized from citrulline in the urea cycle by the sequential action of the cytosolic enzymes argininosuccinate synthetase and argininosuccinate lyase. This is an energetically costly process, because for each molecule of argininosuccinate that is synthesized, one molecule of adenosine triphosphate (ATP) is hydrolyzed to adenosine monophosphate (AMP), consuming two ATP equivalents.
The pathways linking arginine, glutamine, and proline are bidirectional. Thus, the net use or production of these amino acids is highly dependent on cell type and developmental stage.
Arginine is made by the body as follows. The epithelial cells of the small intestine produce citrulline, primarily from glutamine and glutamate, which is secreted into the bloodstream which carries it to the proximal tubule cells of the kidney, which extract the citrulline and convert it to arginine, which is returned to the blood. This means that impaired small bowel or renal function can reduce arginine synthesis and thus create a dietary requirement for arginine. For such a person, arginine would become «essential».
Synthesis of arginine from citrulline also occurs at a low level in many other cells, and cellular capacity for arginine synthesis can be markedly increased under circumstances that increase the production of inducible nitric oxide synthase (NOS). This allows citrulline, a byproduct of the NOS-catalyzed production of nitric oxide, to be recycled to arginine in a pathway known as the citrulline to nitric oxide (citrulline-NO) or arginine-citrulline pathway. This is demonstrated by the fact that, in many cell types, nitric oxide synthesis can be supported to some extent by citrulline, and not just by arginine. This recycling is not quantitative, however, because citrulline accumulates in nitric oxide producing cells along with nitrate and nitrite, the stable end-products of nitric oxide breakdown.[23]
Function[edit]
Arginine plays an important role in cell division, wound healing, removing ammonia from the body, immune function,[24] and the release of hormones.[13][25][26] It is a precursor for the synthesis of nitric oxide (NO),[27] making it important in the regulation of blood pressure.[28][29] Arginine is necessary for T-Cells to function in the body, and can lead to their deregulation if depleted.[30][31]
Proteins[edit]
Arginine’s side chain is amphipathic, because at physiological pH it contains a positively charged guanidinium group, which is highly polar, at the end of a hydrophobic aliphatic hydrocarbon chain. Because globular proteins have hydrophobic interiors and hydrophilic surfaces,[32] arginine is typically found on the outside of the protein, where the hydrophilic head group can interact with the polar environment, for example taking part in hydrogen bonding and salt bridges.[33] For this reason, it is frequently found at the interface between two proteins.[34] The aliphatic part of the side chain sometimes remains below the surface of the protein.[33]
Arginine residues in proteins can be deiminated by PAD enzymes to form citrulline, in a post-translational modification process called citrullination.This is important in fetal development, is part of the normal immune process, as well as the control of gene expression, but is also significant in autoimmune diseases.[35] Another post-translational modification of arginine involves methylation by protein methyltransferases.[36]
Precursor[edit]
Arginine is the immediate precursor of nitric oxide, an important signaling molecule which can act as a second messenger, as well as an intercellular messenger which regulates vasodilation, and also has functions in the immune system’s reaction to infection.
Arginine is also a precursor for urea, ornithine, and agmatine; is necessary for the synthesis of creatine; and can also be used for the synthesis of polyamines (mainly through ornithine and to a lesser degree through agmatine, citrulline, and glutamate.) The presence of asymmetric dimethylarginine (ADMA), a close relative, inhibits the nitric oxide reaction; therefore, ADMA is considered a marker for vascular disease, just as L-arginine is considered a sign of a healthy endothelium.
Structure[edit]
Delocalization of charge in guanidinium group of
l-Arginine
The amino acid side-chain of arginine consists of a 3-carbon aliphatic straight chain, the distal end of which is capped by a guanidinium group, which has a pKa of 13.8,[37] and is therefore always protonated and positively charged at physiological pH. Because of the conjugation between the double bond and the nitrogen lone pairs, the positive charge is delocalized, enabling the formation of multiple hydrogen bonds.
Research[edit]
Growth hormone[edit]
Intravenously administered arginine is used in growth hormone stimulation tests[38] because it stimulates the secretion of growth hormone.[39] A review of clinical trials concluded that oral arginine increases growth hormone, but decreases growth hormone secretion, which is normally associated with exercising.[40] However, a more recent trial reported that although oral arginine increased plasma levels of L-arginine it did not cause an increase in growth hormone.[41]
Herpes-Simplex Virus (Cold sores)[edit]
Research from 1964 into amino acid requirements of herpes simplex virus in human cells indicated that «…the lack of arginine or histidine, and possibly the presence of lysine, would interfere markedly with virus synthesis», but concludes that «no ready explanation is available for any of these observations».[42]
Further medical evidence indicates that «absorbing more arginine may indirectly cause cold sores by disrupting the body’s balance of arginine and another amino acid called lysine.»[43][44]
Further reviews conclude that «lysine’s efficacy for herpes labialis may lie more in prevention than treatment.» and that «the use of lysine for decreasing the severity or duration of outbreaks» is not supported, while further research is needed.[45] A 2017 study concludes that «clinicians could consider advising patients that there is a theoretical role of lysine supplementation in the prevention of herpes simplex sores but the research evidence is insufficient to back this. Patients with cardiovascular or gallbladder disease should be cautioned and warned of the theoretical risks.»[46]
High blood pressure[edit]
A meta-analysis showed that L-arginine reduces blood pressure with pooled estimates of 5.4 mmHg for systolic blood pressure and 2.7 mmHg for diastolic blood pressure.[47]
Supplementation with l-arginine reduces diastolic blood pressure and lengthens pregnancy for women with gestational hypertension, including women with high blood pressure as part of pre-eclampsia. It did not lower systolic blood pressure or improve weight at birth.[48]
Schizophrenia[edit]
Both liquid chromatography and liquid chromatography/mass spectrometric assays have found that brain tissue of deceased people with schizophrenia shows altered arginine metabolism. Assays also confirmed significantly reduced levels of γ-aminobutyric acid (GABA), but increased agmatine concentration and glutamate/GABA ratio in the schizophrenia cases. Regression analysis indicated positive correlations between arginase activity and the age of disease onset and between L-ornithine level and the duration of illness. Moreover, cluster analyses revealed that L-arginine and its main metabolites L-citrulline, L-ornithine and agmatine formed distinct groups, which were altered in the schizophrenia group. Despite this, the biological basis of schizophrenia is still poorly understood, a number of factors, such as dopamine hyperfunction, glutamatergic hypofunction, GABAergic deficits, cholinergic system dysfunction, stress vulnerability and neurodevelopmental disruption, have been linked to the aetiology and/or pathophysiology of the disease.[49]
Raynaud’s phenomenon[edit]
Oral L-arginine has been shown to reverse digital necrosis in Raynaud syndrome[50]
Safety[edit]
L-arginine is recognized as safe (GRAS-status) at intakes of up to 20 grams per day.[51]
See also[edit]
- Arginine glutamate
- AAKG
- Canavanine and canaline are toxic analogs of arginine and ornithine.
References[edit]
- ^ «Nomenclature and Symbolism for Amino Acids and Peptides». IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
- ^ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. «Nomenclature and Symbolism for Amino Acids and Peptides». Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Archived from the original on 29 May 2007. Retrieved 2007-05-17.
- ^ a b c Ignarro LJ (2000-09-13). Nitric Oxide: Biology and Pathobiology. Academic Press. p. 189. ISBN 978-0-08-052503-7.
- ^ Apel F (July 2015). «Biographie von Ernst Schulze» (PDF). Archived from the original (PDF) on 17 November 2015. Retrieved 2017-11-06.
- ^ Schulze E, Steiger E (1887). «Ueber das Arginin» [On arginine]. Zeitschrift für Physiologische Chemie. 11 (1–2): 43–65.
- ^ «BIOETYMOLOGY: ORIGIN IN BIO-MEDICAL TERMS: arginine (Arg R)». Retrieved 25 July 2019.
- ^ Schulze E, Winterstein E (September 1897). «Ueber ein Spaltungs-product des Arginins» [On a cleavage product of arginine]. Berichte der Deutschen Chemischen Gesellschaft (in German). 30 (3): 2879–2882. doi:10.1002/cber.18970300389. The structure for arginine is presented on p. 2882.
- ^ Schulze E, Winterstein E (October 1899). «Ueber die Constitution des Arginins» [On the constitution of arginine]. Berichte der Deutschen Chemischen Gesellschaft (in German). 32 (3): 3191–3194. doi:10.1002/cber.18990320385.
- ^ Cohen JB (1919). Organic Chemistry for Advanced Students, Part 3 (2nd ed.). New York, New York, USA: Longmans, Green & Co. p. 140.
- ^ Sölrensen SP (January 1910). «Über die Synthese des dl-Arginins (α-Amino-δ-guanido-n-valeriansäure) und der isomeren α-Guanido-δ-amino-n-valeriansäure» [On the synthesis of racemic arginine (α-amino-δ-guanido-n-valeric acid) and of the isomeric α-guanido-δ-amino-n-valeric acid]. Berichte der Deutschen Chemischen Gesellschaft (in German). 43 (1): 643–651. doi:10.1002/cber.191004301109.
- ^ Brand E, Sandberg M (1932). «d-Arginine Hydrochloride». Org. Synth. 12: 4. doi:10.15227/orgsyn.012.0004.
- ^ Drauz K, Grayson I, Kleemann A, et al. (2006). «Amino Acids». Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_057.pub2.
- ^ a b Tapiero H, Mathé G, Couvreur P, Tew KD (November 2002). «L-Arginine». (review). Biomedicine & Pharmacotherapy. 56 (9): 439–445. doi:10.1016/s0753-3322(02)00284-6. PMID 12481980.
- ^ Wu G, Jaeger LA, Bazer FW, Rhoads JM (August 2004). «Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications». (review). The Journal of Nutritional Biochemistry. 15 (8): 442–51. doi:10.1016/j.jnutbio.2003.11.010. PMID 15302078.
- ^ «Drugs and Supplements Arginine». Mayo Clinic. Retrieved 15 January 2015.
- ^ Skipper A (1998). Dietitian’s Handbook of Enteral and Parenteral Nutrition. Jones & Bartlett Learning. p. 76. ISBN 978-0-8342-0920-6.
- ^ a b Borlase BC (1994). Enteral Nutrition. Jones & Bartlett Learning. p. 48. ISBN 978-0-412-98471-6.
- ^ Freedland RA, Briggs S (2012-12-06). A Biochemical Approach to Nutrition. Springer Science & Business Media. p. 45. ISBN 9789400957329.
- ^ Nutrient Requirements of Dogs. National Academies Press. 1985. p. 65. ISBN 978-0-309-03496-8.
- ^ a b Wortinger A, Burns K (2015-06-11). Nutrition and Disease Management for Veterinary Technicians and Nurses. John Wiley & Sons. p. 232. ISBN 978-1-118-81108-5.
- ^ Spano MA, Kruskall LJ, Thomas DT (2017-08-30). Nutrition for Sport, Exercise, and Health. Human Kinetics. p. 240. ISBN 978-1-4504-1487-6.
- ^ a b Watson RR, Zibadi S (2012-11-28). Bioactive Dietary Factors and Plant Extracts in Dermatology. Springer Science & Business Media. p. 75. ISBN 978-1-62703-167-7.
- ^ Morris SM (October 2004). «Enzymes of arginine metabolism». (review). The Journal of Nutrition. 134 (10 Suppl): 2743S–2747S, discussion 2765S–2767S. doi:10.1093/jn/134.10.2743S. PMID 15465778.
- ^ Mauro C, Frezza C (2015-07-13). The Metabolic Challenges of Immune Cells in Health and Disease. Frontiers Media SA. p. 17. ISBN 9782889196227.
- ^ Stechmiller JK, Childress B, Cowan L (February 2005). «Arginine supplementation and wound healing». (review). Nutrition in Clinical Practice. 20 (1): 52–61. doi:10.1177/011542650502000152. PMID 16207646.
- ^ Witte MB, Barbul A (2003). «Arginine physiology and its implication for wound healing». (review). Wound Repair and Regeneration. 11 (6): 419–23. doi:10.1046/j.1524-475X.2003.11605.x. PMID 14617280. S2CID 21239136.
- ^ Andrew PJ, Mayer B (August 1999). «Enzymatic function of nitric oxide synthases». (review). Cardiovascular Research. 43 (3): 521–31. doi:10.1016/S0008-6363(99)00115-7. PMID 10690324.
- ^ Gokce N (October 2004). «L-arginine and hypertension». The Journal of Nutrition. 134 (10 Suppl): 2807S–2811S, discussion 2818S–2819S. doi:10.1093/jn/134.10.2807S. PMID 15465790.
- ^ Kibe R, Kurihara S, Sakai Y, et al. (2014). «Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice». Scientific Reports. 4 (4548): 4548. Bibcode:2014NatSR…4E4548K. doi:10.1038/srep04548. PMC 4070089. PMID 24686447.
- ^ Banerjee, Kasturi; Chattopadhyay, Agnibha; Banerjee, Satarupa (2022-07-01). «Understanding the association of stem cells in fetal development and carcinogenesis during pregnancy». Advances in Cancer Biology — Metastasis. 4: 100042. doi:10.1016/j.adcanc.2022.100042. ISSN 2667-3940. S2CID 248485831.
- ^ Rodriguez, Paulo C.; Quiceno, David G.; Ochoa, Augusto C. (2006-10-05). «l-arginine availability regulates T-lymphocyte cell-cycle progression». Blood. 109 (4): 1568–1573. doi:10.1182/blood-2006-06-031856. ISSN 0006-4971. PMC 1794048. PMID 17023580.
- ^ Mathews CK, Van Holde KE, Ahern KG (2000). Biochemistry (3rd ed.). San Francisco, Calif.: Benjamin Cummings. pp. 180. ISBN 978-0805330663. OCLC 42290721.
- ^ a b Barnes MR (2007-04-16). Bioinformatics for Geneticists: A Bioinformatics Primer for the Analysis of Genetic Data. John Wiley & Sons. p. 326. ISBN 9780470026199.
- ^ Kleanthous C (2000). Protein-protein Recognition. Oxford University Press. p. 13. ISBN 9780199637607.
- ^ Griffiths & Unwin 2016, p. 275.
- ^ Griffiths & Unwin 2016, p. 176.
- ^ Fitch CA, Platzer G, Okon M, et al. (May 2015). «Arginine: Its pKa value revisited». Protein Science. 24 (5): 752–61. doi:10.1002/pro.2647. PMC 4420524. PMID 25808204.
- ^ MedlinePlus Encyclopedia: Growth hormone stimulation test
- ^ Alba-Roth J, Müller OA, Schopohl J, von Werder K (December 1988). «Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion». The Journal of Clinical Endocrinology and Metabolism. 67 (6): 1186–9. doi:10.1210/jcem-67-6-1186. PMID 2903866. S2CID 7488757.
- ^ Kanaley JA (January 2008). «Growth hormone, arginine and exercise». Current Opinion in Clinical Nutrition and Metabolic Care. 11 (1): 50–4. doi:10.1097/MCO.0b013e3282f2b0ad. PMID 18090659. S2CID 22842434.
- ^ Forbes SC, Bell GJ (June 2011). «The acute effects of a low and high dose of oral L-arginine supplementation in young active males at rest». Applied Physiology, Nutrition, and Metabolism. 36 (3): 405–11. doi:10.1139/h11-035. PMID 21574873.
- ^ Tankersley RW (March 1964). «Amino Acid Requirements of Herpes Simplex Virus in Human Cells». Journal of Bacteriology. 87 (3): 609–613. doi:10.1128/jb.87.3.609-613.1964. PMC 277062. PMID 14127578.
- ^ «High-arginine foods: Sources, benefits, and risks». www.medicalnewstoday.com. 2018-10-04. Retrieved 2021-05-27.
- ^ «L-Arginine: MedlinePlus Supplements». medlineplus.gov. Retrieved 2021-05-27.
- ^ Tomblin FA, Lucas KH (February 2001). «Lysine for management of herpes labialis». American Journal of Health-System Pharmacy. 58 (4): 298–300, 304. doi:10.1093/ajhp/58.4.298. PMID 11225166.
- ^ Mailoo VJ, Rampes S (June 2017). «Lysine for Herpes Simplex Prophylaxis: A Review of the Evidence». Integrative Medicine. 16 (3): 42–46. PMC 6419779. PMID 30881246.
- ^ Dong JY, Qin LQ, Zhang Z, et al. (December 2011). «Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials». review. American Heart Journal. 162 (6): 959–65. doi:10.1016/j.ahj.2011.09.012. PMID 22137067.
- ^ Gui S, Jia J, Niu X, et al. (March 2014). «Arginine supplementation for improving maternal and neonatal outcomes in hypertensive disorder of pregnancy: a systematic review». (review). Journal of the Renin-Angiotensin-Aldosterone System. 15 (1): 88–96. doi:10.1177/1470320313475910. PMID 23435582.
- ^ Liu P, Jing Y, Collie ND, et al. (August 2016). «Altered brain arginine metabolism in schizophrenia». Translational Psychiatry. 6 (8): e871. doi:10.1038/tp.2016.144. PMC 5022089. PMID 27529679.
- ^ Rembold, Christopher M.; Ayers, Carlos R. (February 2003). «Oral L-arginine can reverse digital necrosis in Raynaud’s phenomenon». Molecular and Cellular Biochemistry. 244 (1–2): 139–141. doi:10.1023/A:1022422932108. ISSN 0300-8177. PMID 12701823. S2CID 30249281.
- ^ Shao A, Hathcock JN (April 2008). «Risk assessment for the amino acids taurine, L-glutamine and L-arginine». Regulatory Toxicology and Pharmacology. 50 (3): 376–99. doi:10.1016/j.yrtph.2008.01.004. PMID 18325648.
Sources[edit]
- Griffiths JR, Unwin RD (2016). Analysis of Protein Post-Translational Modifications by Mass Spectrometry. John Wiley & Sons. ISBN 978-1-119-25088-3.
External links[edit]
Wikimedia Commons has media related to Arginine.
- NIST Chemistry Webbook
- [1]
- National Institute of Health discussion of Arginine.
From Wikipedia, the free encyclopedia

Skeletal formula of arginine |
||
|
||
| Names | ||
|---|---|---|
| IUPAC names
Arginine |
||
| Other names
2-Amino-5-guanidinopentanoic acid |
||
| Identifiers | ||
|
CAS Number |
|
|
|
3D model (JSmol) |
|
|
| 3DMet |
|
|
|
Beilstein Reference |
1725411, 1725412 D, 1725413 L | |
| ChEBI |
|
|
| ChEMBL |
|
|
| ChemSpider |
|
|
| DrugBank |
|
|
| ECHA InfoCard | 100.000.738 |
|
| EC Number |
|
|
|
Gmelin Reference |
364938 D | |
|
IUPHAR/BPS |
|
|
| KEGG |
|
|
| MeSH | Arginine | |
|
PubChem CID |
|
|
| RTECS number |
|
|
| UNII |
|
|
|
CompTox Dashboard (EPA) |
|
|
|
InChI
|
||
|
SMILES
|
||
| Properties | ||
|
Chemical formula |
C6H14N4O2 | |
| Molar mass | 174.204 g·mol−1 | |
| Appearance | White crystals | |
| Odor | Odourless | |
| Melting point | 260 °C; 500 °F; 533 K | |
| Boiling point | 368 °C (694 °F; 641 K) | |
|
Solubility in water |
14.87 g/100 mL (20 °C) | |
| Solubility | slightly soluble in ethanol insoluble in ethyl ether |
|
| log P | −1.652 | |
| Acidity (pKa) | 2.18 (carboxyl), 9.09 (amino), 13.8 (guanidino) | |
| Thermochemistry | ||
|
Heat capacity (C) |
232.8 J K−1 mol−1 (at 23.7 °C) | |
|
Std molar |
250.6 J K−1 mol−1 | |
|
Std enthalpy of |
−624.9–−622.3 kJ mol−1 | |
|
Std enthalpy of |
−3.7396–−3.7370 MJ mol−1 | |
| Pharmacology | ||
|
ATC code |
B05XB01 (WHO) S | |
| Hazards | ||
| GHS labelling: | ||
|
Pictograms |

|
|
|
Signal word |
Warning | |
|
Hazard statements |
H319 | |
|
Precautionary statements |
P305+P351+P338 | |
| Lethal dose or concentration (LD, LC): | ||
|
LD50 (median dose) |
5110 mg/kg (rat, oral) | |
| Safety data sheet (SDS) | L-Arginine | |
| Related compounds | ||
|
Related alkanoic acids |
|
|
|
Related compounds |
|
|
| Supplementary data page | ||
| Arginine (data page) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references |
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the l-arginine (symbol Arg or R) enantiomer is found naturally.[1] Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG.[2] The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide.[3] Like all amino acids, it is a white, water-soluble solid.
History[edit]
Arginine was first isolated in 1886 from yellow lupin seedlings by the German chemist Ernst Schulze and his assistant Ernst Steiger.[4][5] He named it from the Greek árgyros (ἄργυρος) meaning «silver» due to the silver-white appearance of arginine nitrate crystals.[6] In 1897, Schulze and Ernst Winterstein (1865–1949) determined the structure of arginine.[7] Schulze and Winterstein synthesized arginine from ornithine and cyanamide in 1899,[8] but some doubts about arginine’s structure lingered[9] until Sørensen’s synthesis of 1910.[10]
Sources[edit]
Production[edit]
It is traditionally obtained by hydrolysis of various cheap sources of protein, such as gelatin.[11] It is obtained commercially by fermentation. In this way, 25-35 g/liter can be produced, using glucose as a carbon source.[12]
Dietary sources[edit]
Arginine is classified as a semiessential or conditionally essential amino acid, depending on the developmental stage and health status of the individual.[13] Preterm infants are unable to synthesize arginine internally, making the amino acid nutritionally essential for them.[14] Most healthy people do not need to supplement with arginine because it is a component of all protein-containing foods[15] and can be synthesized in the body from glutamine via citrulline.[16][17] Additional dietary arginine is necessary for otherwise healthy individuals temporarily under physiological stress, for example during recovery from burns, injury or sepsis,[17] or if either of the major sites of arginine biosynthesis, the small intestine and kidneys, have reduced function, because the small bowel does the first step of the synthesizing process and the kidneys do the second.[3]
Arginine is an essential amino acid for birds, as they do not have a urea cycle.[18] For some carnivores, for example cats, dogs[19] and ferrets, arginine is essential,[3] because after a meal, their highly efficient protein catabolism produces large quantities of ammonia which need to be processed through the urea cycle, and if not enough arginine is present, the resulting ammonia toxicity can be lethal.[20] This is not a problem in practice, because meat contains sufficient arginine to avoid this situation.[20]
Animal sources of arginine include meat, dairy products, and eggs,[21][22] and plant sources include seeds of all types, for example grains, beans, and nuts.[22]
Biosynthesis[edit]
Arginine is synthesized from citrulline in the urea cycle by the sequential action of the cytosolic enzymes argininosuccinate synthetase and argininosuccinate lyase. This is an energetically costly process, because for each molecule of argininosuccinate that is synthesized, one molecule of adenosine triphosphate (ATP) is hydrolyzed to adenosine monophosphate (AMP), consuming two ATP equivalents.
The pathways linking arginine, glutamine, and proline are bidirectional. Thus, the net use or production of these amino acids is highly dependent on cell type and developmental stage.
Arginine is made by the body as follows. The epithelial cells of the small intestine produce citrulline, primarily from glutamine and glutamate, which is secreted into the bloodstream which carries it to the proximal tubule cells of the kidney, which extract the citrulline and convert it to arginine, which is returned to the blood. This means that impaired small bowel or renal function can reduce arginine synthesis and thus create a dietary requirement for arginine. For such a person, arginine would become «essential».
Synthesis of arginine from citrulline also occurs at a low level in many other cells, and cellular capacity for arginine synthesis can be markedly increased under circumstances that increase the production of inducible nitric oxide synthase (NOS). This allows citrulline, a byproduct of the NOS-catalyzed production of nitric oxide, to be recycled to arginine in a pathway known as the citrulline to nitric oxide (citrulline-NO) or arginine-citrulline pathway. This is demonstrated by the fact that, in many cell types, nitric oxide synthesis can be supported to some extent by citrulline, and not just by arginine. This recycling is not quantitative, however, because citrulline accumulates in nitric oxide producing cells along with nitrate and nitrite, the stable end-products of nitric oxide breakdown.[23]
Function[edit]
Arginine plays an important role in cell division, wound healing, removing ammonia from the body, immune function,[24] and the release of hormones.[13][25][26] It is a precursor for the synthesis of nitric oxide (NO),[27] making it important in the regulation of blood pressure.[28][29] Arginine is necessary for T-Cells to function in the body, and can lead to their deregulation if depleted.[30][31]
Proteins[edit]
Arginine’s side chain is amphipathic, because at physiological pH it contains a positively charged guanidinium group, which is highly polar, at the end of a hydrophobic aliphatic hydrocarbon chain. Because globular proteins have hydrophobic interiors and hydrophilic surfaces,[32] arginine is typically found on the outside of the protein, where the hydrophilic head group can interact with the polar environment, for example taking part in hydrogen bonding and salt bridges.[33] For this reason, it is frequently found at the interface between two proteins.[34] The aliphatic part of the side chain sometimes remains below the surface of the protein.[33]
Arginine residues in proteins can be deiminated by PAD enzymes to form citrulline, in a post-translational modification process called citrullination.This is important in fetal development, is part of the normal immune process, as well as the control of gene expression, but is also significant in autoimmune diseases.[35] Another post-translational modification of arginine involves methylation by protein methyltransferases.[36]
Precursor[edit]
Arginine is the immediate precursor of nitric oxide, an important signaling molecule which can act as a second messenger, as well as an intercellular messenger which regulates vasodilation, and also has functions in the immune system’s reaction to infection.
Arginine is also a precursor for urea, ornithine, and agmatine; is necessary for the synthesis of creatine; and can also be used for the synthesis of polyamines (mainly through ornithine and to a lesser degree through agmatine, citrulline, and glutamate.) The presence of asymmetric dimethylarginine (ADMA), a close relative, inhibits the nitric oxide reaction; therefore, ADMA is considered a marker for vascular disease, just as L-arginine is considered a sign of a healthy endothelium.
Structure[edit]
Delocalization of charge in guanidinium group of
l-Arginine
The amino acid side-chain of arginine consists of a 3-carbon aliphatic straight chain, the distal end of which is capped by a guanidinium group, which has a pKa of 13.8,[37] and is therefore always protonated and positively charged at physiological pH. Because of the conjugation between the double bond and the nitrogen lone pairs, the positive charge is delocalized, enabling the formation of multiple hydrogen bonds.
Research[edit]
Growth hormone[edit]
Intravenously administered arginine is used in growth hormone stimulation tests[38] because it stimulates the secretion of growth hormone.[39] A review of clinical trials concluded that oral arginine increases growth hormone, but decreases growth hormone secretion, which is normally associated with exercising.[40] However, a more recent trial reported that although oral arginine increased plasma levels of L-arginine it did not cause an increase in growth hormone.[41]
Herpes-Simplex Virus (Cold sores)[edit]
Research from 1964 into amino acid requirements of herpes simplex virus in human cells indicated that «…the lack of arginine or histidine, and possibly the presence of lysine, would interfere markedly with virus synthesis», but concludes that «no ready explanation is available for any of these observations».[42]
Further medical evidence indicates that «absorbing more arginine may indirectly cause cold sores by disrupting the body’s balance of arginine and another amino acid called lysine.»[43][44]
Further reviews conclude that «lysine’s efficacy for herpes labialis may lie more in prevention than treatment.» and that «the use of lysine for decreasing the severity or duration of outbreaks» is not supported, while further research is needed.[45] A 2017 study concludes that «clinicians could consider advising patients that there is a theoretical role of lysine supplementation in the prevention of herpes simplex sores but the research evidence is insufficient to back this. Patients with cardiovascular or gallbladder disease should be cautioned and warned of the theoretical risks.»[46]
High blood pressure[edit]
A meta-analysis showed that L-arginine reduces blood pressure with pooled estimates of 5.4 mmHg for systolic blood pressure and 2.7 mmHg for diastolic blood pressure.[47]
Supplementation with l-arginine reduces diastolic blood pressure and lengthens pregnancy for women with gestational hypertension, including women with high blood pressure as part of pre-eclampsia. It did not lower systolic blood pressure or improve weight at birth.[48]
Schizophrenia[edit]
Both liquid chromatography and liquid chromatography/mass spectrometric assays have found that brain tissue of deceased people with schizophrenia shows altered arginine metabolism. Assays also confirmed significantly reduced levels of γ-aminobutyric acid (GABA), but increased agmatine concentration and glutamate/GABA ratio in the schizophrenia cases. Regression analysis indicated positive correlations between arginase activity and the age of disease onset and between L-ornithine level and the duration of illness. Moreover, cluster analyses revealed that L-arginine and its main metabolites L-citrulline, L-ornithine and agmatine formed distinct groups, which were altered in the schizophrenia group. Despite this, the biological basis of schizophrenia is still poorly understood, a number of factors, such as dopamine hyperfunction, glutamatergic hypofunction, GABAergic deficits, cholinergic system dysfunction, stress vulnerability and neurodevelopmental disruption, have been linked to the aetiology and/or pathophysiology of the disease.[49]
Raynaud’s phenomenon[edit]
Oral L-arginine has been shown to reverse digital necrosis in Raynaud syndrome[50]
Safety[edit]
L-arginine is recognized as safe (GRAS-status) at intakes of up to 20 grams per day.[51]
See also[edit]
- Arginine glutamate
- AAKG
- Canavanine and canaline are toxic analogs of arginine and ornithine.
References[edit]
- ^ «Nomenclature and Symbolism for Amino Acids and Peptides». IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
- ^ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. «Nomenclature and Symbolism for Amino Acids and Peptides». Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Archived from the original on 29 May 2007. Retrieved 2007-05-17.
- ^ a b c Ignarro LJ (2000-09-13). Nitric Oxide: Biology and Pathobiology. Academic Press. p. 189. ISBN 978-0-08-052503-7.
- ^ Apel F (July 2015). «Biographie von Ernst Schulze» (PDF). Archived from the original (PDF) on 17 November 2015. Retrieved 2017-11-06.
- ^ Schulze E, Steiger E (1887). «Ueber das Arginin» [On arginine]. Zeitschrift für Physiologische Chemie. 11 (1–2): 43–65.
- ^ «BIOETYMOLOGY: ORIGIN IN BIO-MEDICAL TERMS: arginine (Arg R)». Retrieved 25 July 2019.
- ^ Schulze E, Winterstein E (September 1897). «Ueber ein Spaltungs-product des Arginins» [On a cleavage product of arginine]. Berichte der Deutschen Chemischen Gesellschaft (in German). 30 (3): 2879–2882. doi:10.1002/cber.18970300389. The structure for arginine is presented on p. 2882.
- ^ Schulze E, Winterstein E (October 1899). «Ueber die Constitution des Arginins» [On the constitution of arginine]. Berichte der Deutschen Chemischen Gesellschaft (in German). 32 (3): 3191–3194. doi:10.1002/cber.18990320385.
- ^ Cohen JB (1919). Organic Chemistry for Advanced Students, Part 3 (2nd ed.). New York, New York, USA: Longmans, Green & Co. p. 140.
- ^ Sölrensen SP (January 1910). «Über die Synthese des dl-Arginins (α-Amino-δ-guanido-n-valeriansäure) und der isomeren α-Guanido-δ-amino-n-valeriansäure» [On the synthesis of racemic arginine (α-amino-δ-guanido-n-valeric acid) and of the isomeric α-guanido-δ-amino-n-valeric acid]. Berichte der Deutschen Chemischen Gesellschaft (in German). 43 (1): 643–651. doi:10.1002/cber.191004301109.
- ^ Brand E, Sandberg M (1932). «d-Arginine Hydrochloride». Org. Synth. 12: 4. doi:10.15227/orgsyn.012.0004.
- ^ Drauz K, Grayson I, Kleemann A, et al. (2006). «Amino Acids». Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_057.pub2.
- ^ a b Tapiero H, Mathé G, Couvreur P, Tew KD (November 2002). «L-Arginine». (review). Biomedicine & Pharmacotherapy. 56 (9): 439–445. doi:10.1016/s0753-3322(02)00284-6. PMID 12481980.
- ^ Wu G, Jaeger LA, Bazer FW, Rhoads JM (August 2004). «Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications». (review). The Journal of Nutritional Biochemistry. 15 (8): 442–51. doi:10.1016/j.jnutbio.2003.11.010. PMID 15302078.
- ^ «Drugs and Supplements Arginine». Mayo Clinic. Retrieved 15 January 2015.
- ^ Skipper A (1998). Dietitian’s Handbook of Enteral and Parenteral Nutrition. Jones & Bartlett Learning. p. 76. ISBN 978-0-8342-0920-6.
- ^ a b Borlase BC (1994). Enteral Nutrition. Jones & Bartlett Learning. p. 48. ISBN 978-0-412-98471-6.
- ^ Freedland RA, Briggs S (2012-12-06). A Biochemical Approach to Nutrition. Springer Science & Business Media. p. 45. ISBN 9789400957329.
- ^ Nutrient Requirements of Dogs. National Academies Press. 1985. p. 65. ISBN 978-0-309-03496-8.
- ^ a b Wortinger A, Burns K (2015-06-11). Nutrition and Disease Management for Veterinary Technicians and Nurses. John Wiley & Sons. p. 232. ISBN 978-1-118-81108-5.
- ^ Spano MA, Kruskall LJ, Thomas DT (2017-08-30). Nutrition for Sport, Exercise, and Health. Human Kinetics. p. 240. ISBN 978-1-4504-1487-6.
- ^ a b Watson RR, Zibadi S (2012-11-28). Bioactive Dietary Factors and Plant Extracts in Dermatology. Springer Science & Business Media. p. 75. ISBN 978-1-62703-167-7.
- ^ Morris SM (October 2004). «Enzymes of arginine metabolism». (review). The Journal of Nutrition. 134 (10 Suppl): 2743S–2747S, discussion 2765S–2767S. doi:10.1093/jn/134.10.2743S. PMID 15465778.
- ^ Mauro C, Frezza C (2015-07-13). The Metabolic Challenges of Immune Cells in Health and Disease. Frontiers Media SA. p. 17. ISBN 9782889196227.
- ^ Stechmiller JK, Childress B, Cowan L (February 2005). «Arginine supplementation and wound healing». (review). Nutrition in Clinical Practice. 20 (1): 52–61. doi:10.1177/011542650502000152. PMID 16207646.
- ^ Witte MB, Barbul A (2003). «Arginine physiology and its implication for wound healing». (review). Wound Repair and Regeneration. 11 (6): 419–23. doi:10.1046/j.1524-475X.2003.11605.x. PMID 14617280. S2CID 21239136.
- ^ Andrew PJ, Mayer B (August 1999). «Enzymatic function of nitric oxide synthases». (review). Cardiovascular Research. 43 (3): 521–31. doi:10.1016/S0008-6363(99)00115-7. PMID 10690324.
- ^ Gokce N (October 2004). «L-arginine and hypertension». The Journal of Nutrition. 134 (10 Suppl): 2807S–2811S, discussion 2818S–2819S. doi:10.1093/jn/134.10.2807S. PMID 15465790.
- ^ Kibe R, Kurihara S, Sakai Y, et al. (2014). «Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice». Scientific Reports. 4 (4548): 4548. Bibcode:2014NatSR…4E4548K. doi:10.1038/srep04548. PMC 4070089. PMID 24686447.
- ^ Banerjee, Kasturi; Chattopadhyay, Agnibha; Banerjee, Satarupa (2022-07-01). «Understanding the association of stem cells in fetal development and carcinogenesis during pregnancy». Advances in Cancer Biology — Metastasis. 4: 100042. doi:10.1016/j.adcanc.2022.100042. ISSN 2667-3940. S2CID 248485831.
- ^ Rodriguez, Paulo C.; Quiceno, David G.; Ochoa, Augusto C. (2006-10-05). «l-arginine availability regulates T-lymphocyte cell-cycle progression». Blood. 109 (4): 1568–1573. doi:10.1182/blood-2006-06-031856. ISSN 0006-4971. PMC 1794048. PMID 17023580.
- ^ Mathews CK, Van Holde KE, Ahern KG (2000). Biochemistry (3rd ed.). San Francisco, Calif.: Benjamin Cummings. pp. 180. ISBN 978-0805330663. OCLC 42290721.
- ^ a b Barnes MR (2007-04-16). Bioinformatics for Geneticists: A Bioinformatics Primer for the Analysis of Genetic Data. John Wiley & Sons. p. 326. ISBN 9780470026199.
- ^ Kleanthous C (2000). Protein-protein Recognition. Oxford University Press. p. 13. ISBN 9780199637607.
- ^ Griffiths & Unwin 2016, p. 275.
- ^ Griffiths & Unwin 2016, p. 176.
- ^ Fitch CA, Platzer G, Okon M, et al. (May 2015). «Arginine: Its pKa value revisited». Protein Science. 24 (5): 752–61. doi:10.1002/pro.2647. PMC 4420524. PMID 25808204.
- ^ MedlinePlus Encyclopedia: Growth hormone stimulation test
- ^ Alba-Roth J, Müller OA, Schopohl J, von Werder K (December 1988). «Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion». The Journal of Clinical Endocrinology and Metabolism. 67 (6): 1186–9. doi:10.1210/jcem-67-6-1186. PMID 2903866. S2CID 7488757.
- ^ Kanaley JA (January 2008). «Growth hormone, arginine and exercise». Current Opinion in Clinical Nutrition and Metabolic Care. 11 (1): 50–4. doi:10.1097/MCO.0b013e3282f2b0ad. PMID 18090659. S2CID 22842434.
- ^ Forbes SC, Bell GJ (June 2011). «The acute effects of a low and high dose of oral L-arginine supplementation in young active males at rest». Applied Physiology, Nutrition, and Metabolism. 36 (3): 405–11. doi:10.1139/h11-035. PMID 21574873.
- ^ Tankersley RW (March 1964). «Amino Acid Requirements of Herpes Simplex Virus in Human Cells». Journal of Bacteriology. 87 (3): 609–613. doi:10.1128/jb.87.3.609-613.1964. PMC 277062. PMID 14127578.
- ^ «High-arginine foods: Sources, benefits, and risks». www.medicalnewstoday.com. 2018-10-04. Retrieved 2021-05-27.
- ^ «L-Arginine: MedlinePlus Supplements». medlineplus.gov. Retrieved 2021-05-27.
- ^ Tomblin FA, Lucas KH (February 2001). «Lysine for management of herpes labialis». American Journal of Health-System Pharmacy. 58 (4): 298–300, 304. doi:10.1093/ajhp/58.4.298. PMID 11225166.
- ^ Mailoo VJ, Rampes S (June 2017). «Lysine for Herpes Simplex Prophylaxis: A Review of the Evidence». Integrative Medicine. 16 (3): 42–46. PMC 6419779. PMID 30881246.
- ^ Dong JY, Qin LQ, Zhang Z, et al. (December 2011). «Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials». review. American Heart Journal. 162 (6): 959–65. doi:10.1016/j.ahj.2011.09.012. PMID 22137067.
- ^ Gui S, Jia J, Niu X, et al. (March 2014). «Arginine supplementation for improving maternal and neonatal outcomes in hypertensive disorder of pregnancy: a systematic review». (review). Journal of the Renin-Angiotensin-Aldosterone System. 15 (1): 88–96. doi:10.1177/1470320313475910. PMID 23435582.
- ^ Liu P, Jing Y, Collie ND, et al. (August 2016). «Altered brain arginine metabolism in schizophrenia». Translational Psychiatry. 6 (8): e871. doi:10.1038/tp.2016.144. PMC 5022089. PMID 27529679.
- ^ Rembold, Christopher M.; Ayers, Carlos R. (February 2003). «Oral L-arginine can reverse digital necrosis in Raynaud’s phenomenon». Molecular and Cellular Biochemistry. 244 (1–2): 139–141. doi:10.1023/A:1022422932108. ISSN 0300-8177. PMID 12701823. S2CID 30249281.
- ^ Shao A, Hathcock JN (April 2008). «Risk assessment for the amino acids taurine, L-glutamine and L-arginine». Regulatory Toxicology and Pharmacology. 50 (3): 376–99. doi:10.1016/j.yrtph.2008.01.004. PMID 18325648.
Sources[edit]
- Griffiths JR, Unwin RD (2016). Analysis of Protein Post-Translational Modifications by Mass Spectrometry. John Wiley & Sons. ISBN 978-1-119-25088-3.
External links[edit]
Wikimedia Commons has media related to Arginine.
- NIST Chemistry Webbook
- [1]
- National Institute of Health discussion of Arginine.
аргинин
→
аргинин — существительное, именительный п., муж. p., ед. ч.
↳
аргинин — существительное, винительный п., муж. p., ед. ч.
Часть речи: существительное
| Единственное число | Множественное число | |
|---|---|---|
| Им. |
аргинин |
аргинины |
| Рд. |
аргинина |
аргининов |
| Дт. |
аргинину |
аргининам |
| Вн. |
аргинин |
аргинины |
| Тв. |
аргинином |
аргининами |
| Пр. |
аргинине |
аргининах |
Если вы нашли ошибку, пожалуйста, выделите фрагмент текста и нажмите Ctrl+Enter.
Аргинин
⇒ Правильное написание:
аргинин
⇒ Гласные буквы в слове:
аргинин
гласные выделены красным
гласными являются: а, и, и
общее количество гласных: 3 (три)
• ударная гласная:
аргини́н
ударная гласная выделена знаком ударения « ́»
ударение падает на букву: и
• безударные гласные:
аргинин
безударные гласные выделены пунктирным подчеркиванием « »
безударными гласными являются: а, и
общее количество безударных гласных: 2 (две)
⇒ Согласные буквы в слове:
аргинин
согласные выделены зеленым
согласными являются: р, г, н, н
общее количество согласных: 4 (четыре)
• звонкие согласные:
аргинин
звонкие согласные выделены одинарным подчеркиванием « »
звонкими согласными являются: р, г, н, н
общее количество звонких согласных: 4 (четыре)
⇒ Формы слова:
аргини́н, -а
⇒ Количество букв и слогов:
гласных букв: 3 (три)
согласных букв: 4 (четыре)
всего букв: 7 (семь)
всего слогов: 3 (три)
.
Аргинин
(Argininum)
Содержание
- Структурная формула
- Русское название
- Английское название
- Латинское название вещества Аргинин
- Химическое название
- Брутто формула
- Фармакологическая группа вещества Аргинин
- Нозологическая классификация
- Код CAS
- Торговые названия с действующим веществом Аргинин
Структурная формула
Английское название
Arginine
Латинское название вещества Аргинин
Argininum (род. Arginini)
Химическое название
L-Аргинин
Фармакологическая группа вещества Аргинин
Торговые названия с действующим веществом Аргинин
| Торговое название | Цена за упаковку, руб. |
|---|---|
| L-Аргинин |
1409.00 |
Наш сайт использует файлы cookie, чтобы улучшить работу сайта, повысить его эффективность и удобство. Продолжая использовать сайт rlsnet.ru, вы соглашаетесь с условиями использования файлов cookie.
Аргинин (2-амино-5-гуанидинпентановая кислота) — алифатическая основная α-аминокислота. Оптически активна, существует в виде L- и D- изомеров. L-Аргинин входит в состав пептидов и белков, особенно высоко содержание аргинина в основных белках — гистонах и протаминах (до 85 %). Новое исследование позволяет предположить, что чрезмерное потребление аргинина иммунными клетками, которые обычно защищают мозг, является причиной возникновения болезни Альцгеймера.
Все значения слова «аргинин»
-
В организмах больных кроликов было гораздо больше аргиназы и меньше аргинина, чем у здоровых животных.
-
Таким образом, аргинин представляет собой источник для образования окисид азота.
-
Белки кедровых орехов характеризуются повышенным содержанием аминокислот, и среди них преобладает аргинин – до 20 %.
- (все предложения)
- аминокислота
- лизин
- амигдалин
- бета-каротин
- карнитин
- (ещё синонимы…)
- Склонение
существительного «аргинин»
- Как правильно пишется слово «аргинин»
Аргинин или L-аргинин – одна из 20 аминокислот, необходимых для правильного функционирования организма. Эта аминокислота может быть произведена в организме из глутаминовой кислоты и пролина. В этом отношении эта взаимосвязь носит эндогенный характер. Однако бывает, что в исключительных ситуациях организм теряет способность синтезировать L-аргинин, поэтому необходимо увеличить его поступление с пищей.
Что такое аминокислоты
Элементарные компоненты нашего тела – это белки, состоящие из аминокислот. В организме взрослого человека весом 70 кг содержится около 10 кг белков, что дает нам представление о силе и масштабах действия этих соединений на наш организм.
Аминокислоты – основные компоненты белков. В природе насчитывается более 300 видов природных, а в клетках живых существ их 20. По месту образования аминокислоты можно разделить на эндогенные, то есть вырабатываемые нашим организмом, и экзогенные, поступающие с пищей.
Основной завод, на котором они производятся, – это печень. Именно здесь некоторые аминокислоты, полученные из пищи или белков организма, расщепляются, и в то же время производятся другие, которые используются для синтеза белка. Многие относительно редкие генетические заболевания, такие как фенилкетонурия, связаны с нарушением катаболизма аминокислот, и это заболевание, если его не лечить, может вызвать умственную отсталость или гибель.
Аминокислоты, встречающиеся в природе, используются в качестве лекарств в случае нарушения метаболизма белков в результате неправильного всасывания или потери белка из-за хронических заболеваний, хирургического вмешательства или цирроза печени.
Аргинин достоин Нобелевской премии
Одна из самых важных аминокислот, о которой в последнее время говорят и пишут, – это аргинин. Он вырабатывается в нашем организме в условиях ненарушенного гомеостаза и, следовательно, принадлежит к группе эндогенных аминокислот. Сила его воздействия на организм подтверждается тем фактом, что три американских фармаколога: Роберт Ферчготт, Луи I. Игнарро и Ферид Мурад получили Нобелевскую премию за исследования влияния аргинина на здоровье.
Было обнаружено, что благодаря уникальному механизму действия, аргинин приводит к выделению оксида азота в организме. Это вещество оказывает чрезвычайно благотворное влияние на кровеносные сосуды, и в частности на сосудистый эндотелий. Дефицит оксида азота наблюдается при таких заболеваниях, как атеросклероз, диабет, гипертония или почечная недостаточность.
Таким образом, можно сделать вывод, что аргинин влияет на такие заболевания, как гипертония. Он также:
- подавляет агрегацию тромбоцитов;
- стимулирует фибринолиз;
- стимулирует рост;
- поддерживает метаболизм жиров;
- участвует в передаче сигналов как внутри клетки, так и между клетками.
Доказано, что аргинин положительно влияет на организм при заболеваниях печени, вызванных нарушением цикла мочевины.
Последствия дефицита L-аргинина
Аргинин рассматривают как условно незаменимую аминокислоту, что означает необходимость обеспечения ее соответствующего уровня в организме. Но важно понимать, что дефицит L-аргинина – относительно редкое состояние в первую очередь потому, что организм имеет способность производить его самостоятельно.
Однако доказано, что эффективность его создания по мере естественного развития процесса старения снижается. У недоношенных детей также нарушена функция синтеза L-аргинина. Дефицит этой аминокислоты также может проявляться при истощении организма в результате неправильного питания.
Недостаток L-аргинина в организме может привести к дисбалансу секреции инсулина и нарушению метаболизм жиров в печени. Недостаток аминокислот также приводит к нарушениям в мышечной ткани, а при длительном дефиците – даже к ее исчезновению.
В связи с тем, что аргинин влияет на синтез NO – ключевой молекулы для правильного функционирования кровеносной системы – его недостаток может привести к сердечным заболеваниям.
Аргинин – противопоказания
Есть ли у аргинина противопоказания? Добавки и продукты с этим элементом не рекомендуются при следующих патологиях и состояниях:
- обструкция желчевыводящих путей;
- гипертония высокой степени;
- расстройства пищеварения;
- гиперчувствительность;
- нарушение функции почек;
- печеночная недостаточность.
Аргинин не следует применять людям, страдающим шизофренией, беременным женщинам и кормящим матерям.
Длительное употребление добавок с этой аминокислотой приводит к выработке чрезмерного количества оксида азота, что увеличивает кровяное давление и снижает эффективность работы сердечной мышцы. Также могут возникнуть проблемы с желудком.
Аргинин – дозировка
Рекомендуемая суточная норма аргинина: от 3 до 8 граммов. Для наилучшего использования свойств аргинина рекомендуется принимать его за 2 часа до запланированной физической активности или перед сном.
Источники L-аргинина
Определенное количество L-аргинина вырабатывается организмом человека при условии, что в организме есть достаточное количество пролина и глутаминовой кислоты, из которых он синтезируется. Однако этих количеств может быть недостаточно для правильного протекания обменных процессов. По этой причине трудно четко определить, является ли L-аргинин эндогенной или экзогенной аминокислотой.
Но человеческий организм может поглощать L-аргинин из пищи. Это соединение можно найти как в продуктах животного происхождения, так и в растительных. Богатые источники L-аргинина – молочные продукты, морепродукты, а также белое и красное мясо. Вегетарианцы могут найти его во всех типах орехов и семян, цельнозерновых продуктах, мюсли, нуте и соевых бобах.
Сбалансированная диета не требует приема L-аргинина, так как он хорошо усваивается из пищи.
Безопасны ли добавки аргинина?
Те, кто хочет принимать L-аргинин в качестве добавки, должны проконсультироваться с врачом о потенциальных преимуществах и рисках приема препаратов. Следует исключить возникновение заболеваний, которые могут усугубить прием добавки.
В клинических испытаниях аргинин безопасно использовался с небольшими побочными эффектами только на срок до трех месяцев. Возможные побочные эффекты включали боль в животе, газы, диарею и подагру. Искусственный аргинин также может ухудшить дыхание у людей, страдающих астмой.