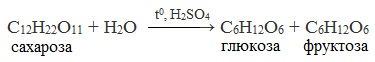Химия,
вопрос задал gffyjdt,
5 лет назад
как пишется сахар по химии
Ответы на вопрос
Ответил kuzmenkon755
0
Ответ:
C12H22O11
Объяснение:
Сахаро́за (сукро́за, тростниковый сахар) C12H22O11, в быту просто сахар, — дисахарид из группы олигосахаридов, состоящий из двух моносахаридов: α-глюкозы и β-фруктозы. Сахароза является весьма распространённым в природе дисахаридом. Она встречается во многих фруктах, плодах и ягодах.
Предыдущий вопрос
Следующий вопрос
Новые вопросы
Қазақ тiлi,
7 месяцев назад
2. Жетісу туралы өлеңді (23, mp3) тыңдап, Жетісудың табиғатын сипатт…
Русский язык,
7 месяцев назад
3. Опиши зайчика. Составь и запиши словосочетания по тексту. Главное слово в каждом словосочетании обозначь знаком Х. Ушки (какие?) …, ножки (какие?) …, шубка (какая?) …, наряд (какой?) …,…
Биология,
5 лет назад
За яких умов популяції будуть репродуктивно ізольованими…
Алгебра,
5 лет назад
Докажите неравенство (х+2)в квадрате>_8х…
Геометрия,
6 лет назад
Диагонали прямоугольника ABCD пересекаются в точке О. Найдите угол между диагоналями, если угол ABO=30 градусам…
История,
6 лет назад
особливості буденного життя українських селян…
- Вопросы и Ответы
- Химия
- Как будет сахар по химии
6 просмотров
Как будет сахар по химии
- будет
- сахар
- химии
- 10 — 11 классы
- химия
спросил
22 Март, 18
от
Varasiskili179_zn
(15 баллов)
в категории Химия
|
6 просмотров
оставил комментарий
22 Март, 18
от
Varasiskili179_zn
(15 баллов)
2 Ответы
0 голосов
Лучший ответ
C6 H12 O6 формула глюкозы C12 H22 O11 формула сахара
ответил
22 Март, 18
от
Pavetta_zn
Супер бакалавр
(18.6k баллов)
0 голосов
C12H22o11-формула сахара.
ответил
22 Март, 18
от
Chizhyana_zn
(33 баллов)
Здравствуйте! На сайте Otvet-Master.ru собраны ответы и решения на все виды школьных задач и университетских заданий. Воспользуйтесь поиском решений на сайте или задайте свой вопрос онлайн и абсолютно бесплатно.
Правильный ответ на вопрос 👍 «Как пишется в химии кристаллический сахар и поваренная соль? …» по предмету 📗 Химия. Развернутая система поиска нашего сайта обязательно приведёт вас к нужной информации. Как вариант — оцените ответы на похожие вопросы. Но если вдруг и это не помогло — задавайте свой вопрос знающим оппонентам, которые быстро дадут на него ответ!
Искать готовые ответы
При сжигании этана образовался углекислый газ объемом 32 л. Какие объемы исходных газов было использовано? (н. у.)
Ответы (1)
Запишите уравнения химических реакций, характеризующие свойства: а) MgO и SO3; б) Mg (OH) 2 и H2SO4. Уравнения реакций с участием электролитов запишите также в ионной форме.
Ответы (1)
Осуществите превращения Cu—CuO—CuSo4—Cu (OH) 2—CuCl2
Ответы (1)
Ксилол 100% консентрация, чем разбавить чтоб получить 40%
Ответы (1)
Запишите схемы образования химических связей для веществ состав которых отображают формулами kcl и cl2
Ответы (1)
Определите степень полимеризации полибутадиена, молекулярная масса которого 13500 г/моль.
Ответы (1)
Дано: BaCl2, NaOH, Na2SO4, AlCl3, HCl, K2CO3, CuSO3, Ba (NO3) 2 Нужно составить возможные реакции ионного обмена.
Ответы (1)
Опишите физические свойства присущие для веществ с молекулярными кристаллическими решетками Приведите примеры
Ответы (1)
Степень окисления J2
Ответы (1)
Определите массовую 10% раствора H2SO4 (плотность раствора H2SO4=1,069 г/мл)
Ответы (1)
Главная » ⭐️ Химия » Как пишется в химии кристаллический сахар и поваренная соль?
Найдите правильный ответ на вопрос ✅ «Как пишется в химии кристаллический сахар и поваренная соль? …» по предмету 📘 Химия, а если вы сомневаетесь в правильности ответов или ответ отсутствует, то попробуйте воспользоваться умным поиском на сайте и найти ответы на похожие вопросы.
Смотреть другие ответы
Главная » ⭐️ Химия » Как пишется в химии кристаллический сахар и поваренная соль?
Лучший ответ
Ирина
Гений
(88052)
12 лет назад
С12Н22О11 сахароза
Остальные ответы
Аня Егорова
Профи
(996)
12 лет назад
В смысле-как?
Формулы сахара в химии нет. Дело в том, что по химической классификации углеводов, сахар — это сладкий и растворимый углевод. Примерами служат глюкоза (виноградный сахар) — С6Н12О6, фруктоза (самый сладкий, плодовый сахар) состав тот же; сахароза (обычный сахар) — С12Н22О11 и другие вещества.
RED
Искусственный Интеллект
(313318)
12 лет назад
Сложное углеродное соединение.
Виктор Щербина
Мудрец
(16106)
12 лет назад
hgdsfhlS — U — в начале пришлось заполнять обязательные поля-Бред модератора
Татьяна Комарова
Ученик
(167)
5 лет назад
Глюкоза (С6Н12О6), глюкоза-это сахар обыкновенный, а не сахороза
knaikear285
Вопрос по химии:
Как пишется в химии кристаллический сахар и поваренная соль?
Трудности с пониманием предмета? Готовишься к экзаменам, ОГЭ или ЕГЭ?
Воспользуйся формой подбора репетитора и занимайся онлайн. Пробный урок — бесплатно!
Ответы и объяснения 1
thias854
Сахар: С12Н22О
Соль: NaCI
Знаете ответ? Поделитесь им!
Гость ?
Как написать хороший ответ?
Как написать хороший ответ?
Чтобы добавить хороший ответ необходимо:
- Отвечать достоверно на те вопросы, на которые знаете
правильный ответ; - Писать подробно, чтобы ответ был исчерпывающий и не
побуждал на дополнительные вопросы к нему; - Писать без грамматических, орфографических и
пунктуационных ошибок.
Этого делать не стоит:
- Копировать ответы со сторонних ресурсов. Хорошо ценятся
уникальные и личные объяснения; - Отвечать не по сути: «Подумай сам(а)», «Легкотня», «Не
знаю» и так далее; - Использовать мат — это неуважительно по отношению к
пользователям; - Писать в ВЕРХНЕМ РЕГИСТРЕ.
Есть сомнения?
Не нашли подходящего ответа на вопрос или ответ отсутствует?
Воспользуйтесь поиском по сайту, чтобы найти все ответы на похожие
вопросы в разделе Химия.
Трудности с домашними заданиями? Не стесняйтесь попросить о помощи —
смело задавайте вопросы!
Химия — одна из важнейших и обширных областей естествознания, наука о веществах, их составе и строении, их свойствах, зависящих от состава и строения, их превращениях, ведущих к изменению состава — химических реакциях, а также о законах и закономерностях, которым эти превращения подчиняются.
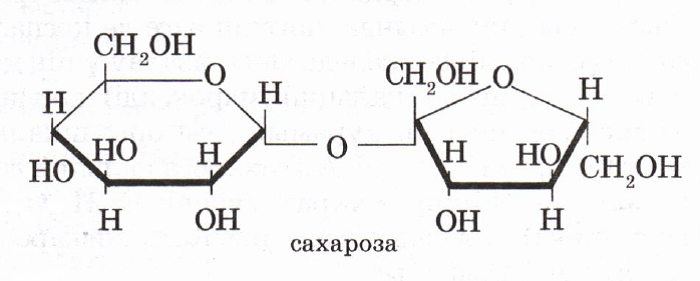
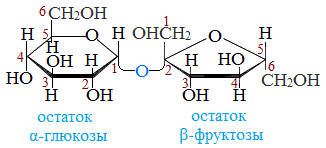
Сахароза — органическое вещество с кристаллической решеткой. Другое название — сахар. Это дисахарид, образованный остатками двух моносахаридов — фруктозы и глюкозы.
Узнаем больше о сахарозе, ее строении, формуле, физических и химических свойствах и о том, какую пользу играет она для живых организмов.
Формула и строение сахарозы
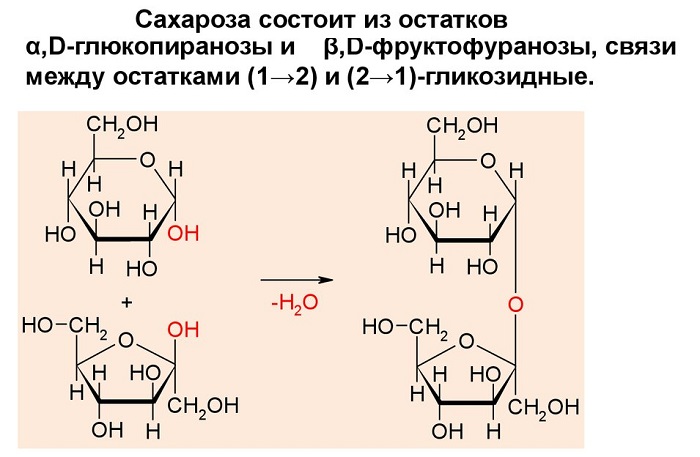
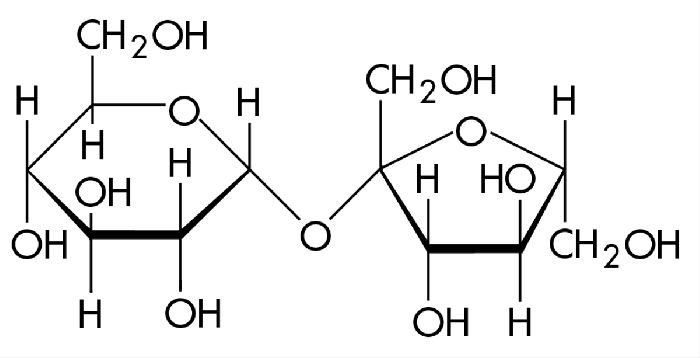
Структурная формула — C12H22O11, хотя она происходит от соединения двух простых сахаров, таких как глюкоза и фруктоза.
Два кольца этих сахаров объединены отдельным атомом кислорода, соединенным с двумя атомами углерода в цепочке. Другое расширение атома в молекуле также присутствует, главным образом, в комбинациях кислорода и водорода.
Связь между моносахаридами имеет O-глюкозидный тип. Кроме того, эта связь является дикарбонильной.
Физические свойства
По своим физическим свойствам она обладает сладким вкусом, может кристаллизоваться и растворима в воде.
Когда сахароза достигает желудка, она подвергается кислотному гидролизу и разлагается на части: на глюкозу и фруктозу. Остальная часть сахарозы переходит в тонкий кишечник, где ферментная сахароза преобразует ее в глюкозу и фруктозу.
Подчеркиваются ее специфические свойства в качестве питательного вещества для организма человека: она легко усваивается и не выделяет токсичных веществ. Это означает, что сахароза обладает как свойствами глюкозы, так и свойствами фруктозы, что означает, что она является источником энергии для организма.
Существует множество противоречий по поводу вреда, наносимого потреблением сахарозы, и несколько теорий по этому поводу. Основные дебаты сосредоточены на развитии кариеса, диабета, ожирения, атеросклероза и других патологий.
Интересно, что сахароза является триболюминесцентной, производящей свет механическим действием.
Благодаря низкой температуре плавления 1860С она очень быстро становится жидкой, очень легко прилипает к контейнеру, в котором она находится, может запросто обжечь кожу, если не соблюдать меры безопасности. Температура кипения раствора равна 101,40С.
Основные свойства описаны в таблице:
|
Физические свойства сахарозы |
|
|
Молярная масса |
342,3 г/моль |
|
Температура плавления |
1860С |
|
Растворимость |
211,5 г / 100 мл |
|
Плотность |
1,587 г/см3 |
Химические свойства
В молекуле сахарозы есть гидроксильные группы. Рассмотрим основные уравнения химических реакций сахарозы.
Реакция гидролиза
Сахароза способна подвергаться гидролизу, во время которого она распадается на моносахариды (глюкозу и фруктозу):
C12H22O11
+ H2O → C6H12O6
+ C6H12O6.
Реакции окисления
Дисахариды, которые сохраняют полуацетальный гидроксил, называются восстанавливающими. Дисахариды без полуацетального гидроксила называются невосстанавлиающими. Сахароза не является альдегидом.
Пример каталитического окисления сахарозы кислородом воздуха:
C12H22O11
+ 12 O2 → 12 CO2 + 11 H2O.
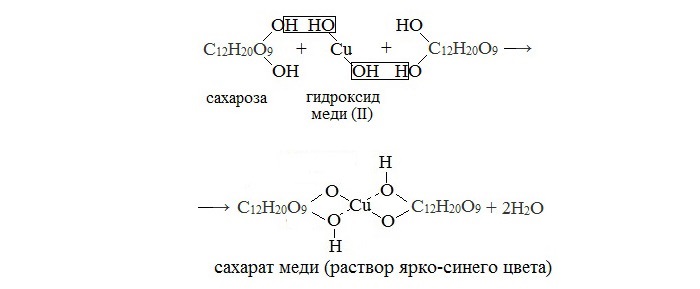
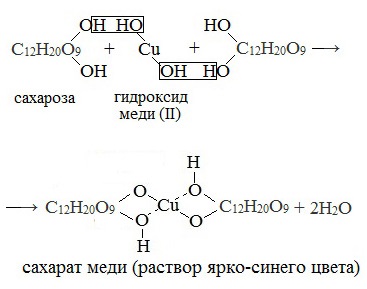
Качественная реакция с гидроксидом меди
Как провести лабораторный опыт:
-
лабораторное оборудование: пробирка, горелка;
-
реагенты: водный раствор сахарозы, гидроксид меди (II);
-
действия: залейте раствор сахарозы в пробирку, добавьте гидроксид меди (II), разогрейте пробирку над горелкой:
C12H22O11
+ 2 Cu (OH)2 → ярко-синее окрашивание;
Замечания: синий осадок не изменил цвет, несмотря на подачу энергии в виде тепла;
Вывод: сахароза не обладает восстановительными свойствами.
Нахождение сахарозы в природе
Она обычно извлекается из сахарного тростника, свеклы или кукурузы.
Сахарный тростник
Другими коммерческими (незначительными) источниками являются сладкий сорго и кленовый сироп.
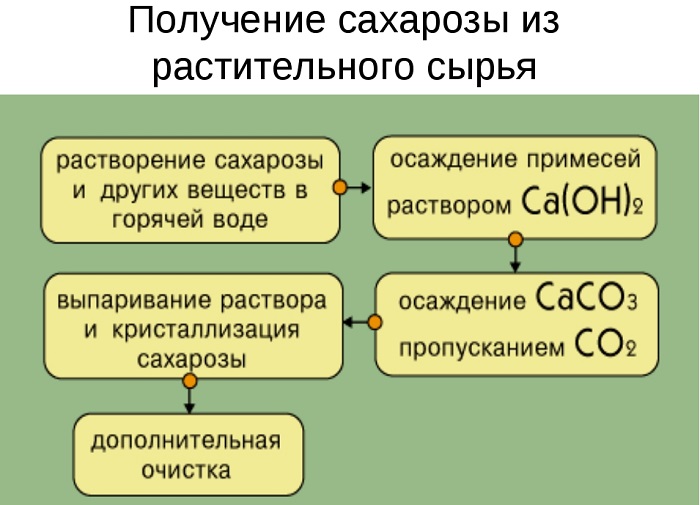
Получение сахарозы
Сахароза извлекается из сырья, в котором она содержится, а затем очищается и кристаллизуется.
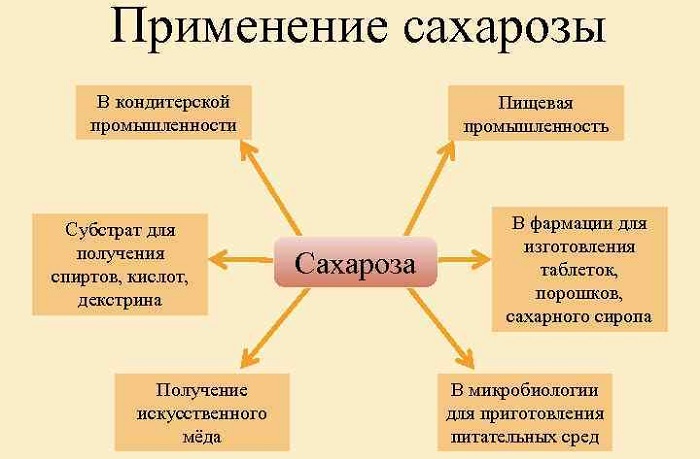
Применение и биологическая роль сахарозы
Широкое применение сахарозы обусловлено ее способностью к подслащению и свойствами функциональной консистенции. По этой причине она важна для структуры некоторых продуктов питания, особенно кондитерских изделий.
Также является вспомогательным компонентом в сохранении пищи, будучи добавкой, широко используемой в приготовлении так называемой нездоровой пищи.
В проросших семенах растений жиры и белки, находящиеся на хранении, превращаются в сахарозу для транспортировки в процессе развития растений.
Главная функция сахарозы в организме человека — она помогает вырабатывать энергию, необходимую для функционирования различных органов.
Эта статья — о химическом веществе. О пищевом продукте см. Сахар.
| Сахароза | |
 |
|
 |
|
| Общие | |
|---|---|
| Систематическое наименование | (2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-дигидрокси-2,5-бис(гидроксиметил)оксолан-2-ил]окси-6-(гидроксиметил)оксан-3,4,5-триол |
| Традиционные названия | α-D-глюкопиранозил-β-D-фруктофуранозид |
| Химическая формула | C12H22O11 |
| Физические свойства | |
| Молярная масса | 342,3 г/моль |
| Плотность | 1,587 г/см³ |
| Термические свойства | |
| Температура плавления | 186 °C |
| Химические свойства | |
| Растворимость в воде | 211,5 г/100 мл |
| Классификация | |
| Рег. номер CAS | 57-50-1 |
| SMILES | OC1C(OC(CO)C(O)C1O) OC2(CO)OC(CO)C(O)C2O |
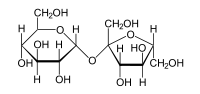
Сахароза C12H22O11, или свекловичный сахар, тростниковый сахар, в быту просто сахар — дисахарид из группы олигосахаридов, состоящий из двух моносахаридов — α-глюкозы и β-фруктозы.
Сахароза является весьма распространённым в природе дисахаридом, она встречается во многих фруктах, плодах и ягодах. Особенно велико содержание сахарозы в сахарной свёкле и сахарном тростнике, которые и используются для промышленного производства пищевого сахара.
Сахароза имеет высокую растворимость. В химическом отношении сахароза довольно инертна, так как при перемещении из одного места в другое почти не вовлекается в метаболизм. Иногда сахароза откладывается в качестве запасного питательного вещества.
Сахароза, попадая в кишечник, быстро гидролизуется альфа-глюкозидазой тонкой кишки на глюкозу и фруктозу, которые затем всасываются в кровь. Ингибиторы альфа-глюкозидазы, такие, как акарбоза, тормозят расщепление и всасывание сахарозы, а также и других углеводов, гидролизуемых альфа-глюкозидазой, в частности, крахмала. Это используется в лечении сахарного диабета 2-го типа[1].
Синонимы: α-D-глюкопиранозил-β-D-фруктофуранозид, свекловичный сахар, тростниковый сахар
Содержание
- 1 Внешний вид
- 2 Химические и физические свойства
- 2.1 Реакция сахарозы с водой
- 2.2 Реакция с гидроксидом меди (II)
- 3 Природные и антропогенные источники
- 4 Галерея
- 5 Примечания
Внешний вид
Кристаллы сахарозы
Бесцветные моноклинные кристаллы. При застывании расплавленной сахарозы образуется аморфная прозрачная масса — карамель.
Химические и физические свойства
Молекулярная масса 342,3 а. е. м. Брутто-формула (система Хилла): C12H22O11. Вкус сладковатый. Растворимость (в граммах на 100 граммов растворителя): в воде 179 (0 °C) и 487 (100 °C), в этаноле 0,9 (20 °C). Малорастворима в метаноле. Не растворима в диэтиловом эфире. Плотность 1,5879 г/см3 (15 °C). Удельное вращение для D-линии натрия: 66,53 (вода; 35 г/100г; 20 °C). При охлаждении жидким воздухом, после освещения ярким светом кристаллы сахарозы фосфоресцируют. Не проявляет восстанавливающих свойств — не реагирует с реактивом Толленса и реактивом Фелинга. Не образует открытую форму, поэтому не проявляет свойств альдегидов и кетонов. Наличие гидроксильных групп в молекуле сахарозы легко подтверждается реакцией с гидроксидами металлов. Если раствор сахарозы прилить к гидроксиду меди (II), образуется ярко-синий раствор сахарата меди. Альдегидной группы в сахарозе нет: при нагревании с аммиачным раствором оксида серебра (I) она не дает «серебряного зеркала», при нагревании с гидроксидом меди (II) не образует красного оксида меди (I). Из числа изомеров сахарозы, имеющих молекулярную формулу С12Н22О11, можно выделить мальтозу и лактозу.
Реакция сахарозы с водой
Если прокипятить раствор сахарозы с несколькими каплями соляной или серной кислоты и нейтрализовать кислоту щелочью, а после этого нагреть раствор, то появляются молекулы с альдегидными группами, которые и восстанавливают гидроксид меди (II) до оксида меди (I). Эта реакция показывает, что сахароза при каталитическом действии кислоты подвергается гидролизу, в результате чего образуются глюкоза и фруктоза:
Реакция с гидроксидом меди (II)
В молекуле сахарозы имеется несколько гидроксильных групп. Поэтому соединение взаимодействует с гидроксидом меди (II) аналогично глицерину и глюкозе. При добавлении раствора сахарозы к осадку гидроксида меди (II) он растворяется; жидкость окрашивается в синий цвет. Но, в отличие от глюкозы, сахароза не восстанавливает гидроксид меди (II) до оксида меди (I).
Природные и антропогенные источники
Содержится в сахарном тростнике, сахарной свёкле (до 28 % сухого вещества), соках растений и плодах (например, берёзы, клёна, дыни и моркови). Источник получения сахарозы — из свёклы или из тростника, определяют по соотношению содержания стабильных изотопов углерода 12C и 13C. Сахарная свёкла имеет C3-механизм усвоения углекислого газа (через фосфоглицериновую кислоту) и предпочтительно поглощает изотоп 12C; сахарный тростник имеет C4-механизм поглощения углекислого газа (через щавелевоуксусную кислоту) и предпочтительно поглощает изотоп 13C.
Мировое производство в 1990 году — 110 000 000 тонн.
Галерея
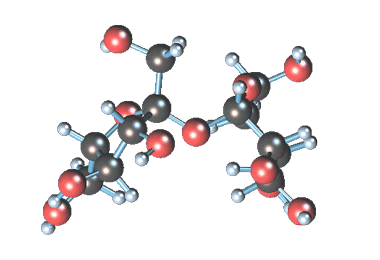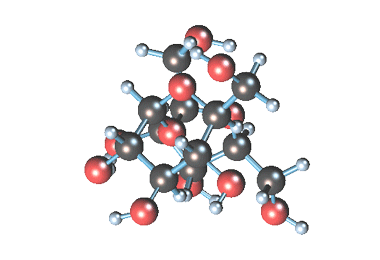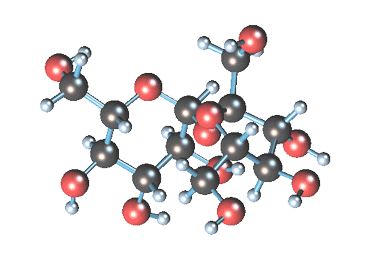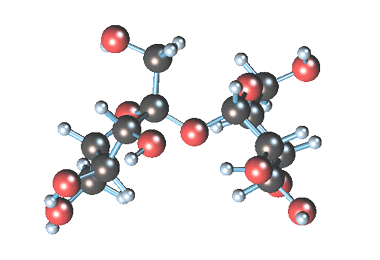
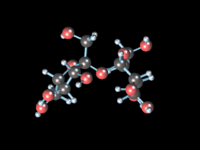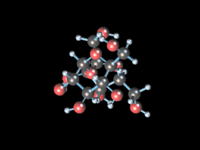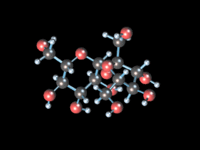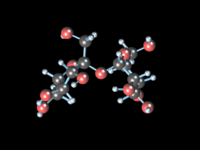
-
Статичное 3D-изображение
молекулы сахарозы. -
Кристаллы коричневого
(тростникового) сахара
Примечания
- ↑ Акарабоза: инструкция по применению.
| |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Общие: | Альдозы · Кетозы · Фуранозы · Пиранозы | ||||||||||||||
| Геометрия | Аномеры · Мутаротация · Проекция Хоуорса | ||||||||||||||
| Моносахариды |
|
||||||||||||||
| Мультисахариды |
|
||||||||||||||
| Производные углеводов |
|
Сахароза

4.3
Средняя оценка: 4.3
Всего получено оценок: 456.
4.3
Средняя оценка: 4.3
Всего получено оценок: 456.
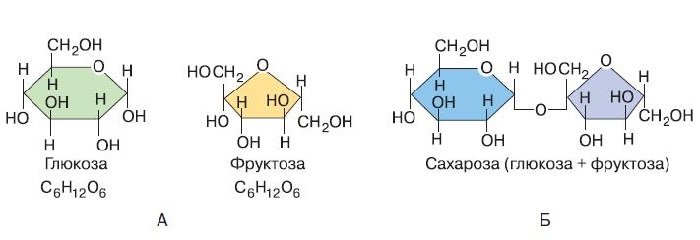
Привычный сладкий сахар, используемый в быту, называется сахарозой. Это олигосахарид, относящийся к группе дисахаридов. Формула сахарозы – C12H22O11.
Строение
В состав молекулы входят остатки двух циклических моносахаридов – α-глюкоза и β-фруктоза. Структурная формула вещества состоит из циклических формул фруктозы и глюкозы, соединённых атомом кислорода. Структурные единицы связаны вместе гликозидной связью, образующейся между двумя гидроксилами.
Молекулы сахарозы образуют молекулярную кристаллическую решетку.
Получение
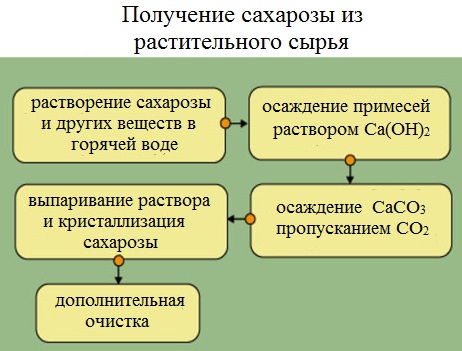
Сахароза – наиболее распространённый в природе углевод. Соединение входит в состав фруктов, ягод, листьев растений. Большое количество готового вещества содержится в свёкле и сахарном тростнике. Поэтому сахарозу не синтезируют, а выделяют с помощью физического воздействия, вываривания и очищения.
Свёклу или сахарный тростник мелко натирают и помещают в большие котлы с горячей водой. Сахароза вымывается, образуя сахарный раствор. В нём присутствуют различные примеси – красящие пигменты, белки, кислоты. Чтобы отделить сахарозу, в раствор добавляют гидроксид кальция Ca(OH)2. В результате образуется осадок и сахарат кальция С12Н22О11·CaO·2Н2О, через который пропускают диоксид углерода (углекислый газ). В осадок выпадает карбонат кальция, а оставшийся раствор выпаривают до образования кристалликов сахара.
Физические свойства
Основные физические характеристики вещества:
- молекулярная масса – 342 г/моль;
- плотность – 1,6 г/см3;
- температура плавления – 186°С.
Если расплавленное вещество продолжить нагревать, сахароза начнёт разлагаться с изменением окраски. При застывании расплавленной сахарозы образуется карамель – аморфное прозрачное вещество. В 100 мл воды при нормальных условиях можно растворить 211,5 г сахара, при 0°С – 176 г, при 100°С – 487 г. В 100 мл этанола при нормальных условиях сахар можно растворить только 0,9 г сахара.
Попадая в кишечник животных и человека, сахароза под действием ферментов быстро распадается на моносахариды.
Химические свойства
В отличие от глюкозы сахароза не проявляет свойства альдегида за счёт отсутствия альдегидной группы -CHO. Поэтому качественная реакция «серебряного зеркала» (взаимодействие с аммиачным раствором Ag2O) не идёт. При окислении гидроксидом меди (II) образуется не красный оксид меди (I), а ярко-синий раствор.
Основные химические свойства описаны в таблице.
|
Реакция |
Описание |
Уравнение |
|
Качественная реакция на наличие гидроксильных групп |
Реагирует с гидроксидом меди (II) с образованием сахарата меди ярко-синего цвета |
C12H22O11 + Cu(OH)2 → [Cu(C12H21O11)2] + Н2О |
|
Гидролиз |
Реакция идёт при нагревании в присутствии катализатора (серной или соляной кислоты). Сахароза разлагается на молекулы фруктозы и глюкозы |
С12Н22О11 + Н2О → C6H12O6 + C6H12O6 |
Сахароза не способна окисляться (не является восстановителем в реакциях) и называется невосстанавливающим сахаром.
Применение
Сахар в чистом виде используется в пищевой промышленности для изготовления искусственного мёда, сладостей, кондитерских изделий, алкоголя. Сахарозу используют для получения различных веществ: лимонной кислоты, глицерина, бутанола.
В медицине сахарозу используют для изготовления микстур и порошков, чтобы скрыть неприятный вкус.
Что мы узнали?
Сахароза или сахар – дисахарид, состоящий из остатков глюкозы и фруктозы. Обладает сладким вкусом, легко растворяется в воде. Вещество выделяют из свёклы и сахарного тростника. Сахароза обладает меньшей активностью, чем глюкоза. Подвергается гидролизу, реагирует с гидроксидом меди (II), образуя сахарат меди, не окисляется. Сахар используют в пищевой, химической промышленности, медицине.
Тест по теме
Доска почёта

Чтобы попасть сюда — пройдите тест.
-
Кирилл Плотников
10/10
-
Эля Щербакова
10/10
Оценка доклада
4.3
Средняя оценка: 4.3
Всего получено оценок: 456.
А какая ваша оценка?
Sucrose

|
|

|
|
|
|
| Names | |
|---|---|
| IUPAC name
β-D-Fructofuranosyl α-D-glucopyranoside |
|
| Preferred IUPAC name
(2R,3R,4S,5S,6R)-2-{[(2S,3S,4S,5R)-3,4-Dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|
Other names
|
|
| Identifiers | |
|
CAS Number |
|
|
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.000.304 |
| EC Number |
|
|
IUPHAR/BPS |
|
| KEGG |
|
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
|
CompTox Dashboard (EPA) |
|
|
InChI
|
|
|
SMILES
|
|
| Properties[1] | |
|
Chemical formula |
C 12H 22O 11 |
| Molar mass | 342.30 g/mol |
| Appearance | white solid |
| Density | 1.587 g/cm3 (0.0573 lb/cu in), solid |
| Melting point | None; decomposes at 186°C (367°F; 459 K) |
|
Solubility in water |
~200 g/dL (25 °C (77 °F)) |
| log P | −3.76 |
| Structure | |
|
Crystal structure |
Monoclinic |
|
Space group |
P21 |
| Thermochemistry | |
|
Std enthalpy of |
1,349.6 kcal/mol (5,647 kJ/mol)[2] (Higher heating value) |
| Hazards | |
| NFPA 704 (fire diamond) |
0 1 0 |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose) |
29700 mg/kg (oral, rat)[4] |
| NIOSH (US health exposure limits): | |
|
PEL (Permissible) |
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[3] |
|
REL (Recommended) |
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[3] |
|
IDLH (Immediate danger) |
N.D.[3] |
| Safety data sheet (SDS) | ICSC 1507 |
| Related compounds | |
|
Related compounds |
Lactose Maltose |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula C
12H
22O
11.
For human consumption, sucrose is extracted and refined from either sugarcane or sugar beet. Sugar mills – typically located in tropical regions near where sugarcane is grown – crush the cane and produce raw sugar which is shipped to other factories for refining into pure sucrose. Sugar beet factories are located in temperate climates where the beet is grown, and process the beets directly into refined sugar. The sugar-refining process involves washing the raw sugar crystals before dissolving them into a sugar syrup which is filtered and then passed over carbon to remove any residual colour. The sugar syrup is then concentrated by boiling under a vacuum and crystallized as the final purification process to produce crystals of pure sucrose that are clear, odorless, and sweet.
Sugar is often an added ingredient in food production and recipes. About 185 million tonnes of sugar were produced worldwide in 2017.[5]
Sucrose is particularly dangerous as a risk factor for tooth decay because Streptococcus mutans bacteria convert it into a sticky, extracellular, dextran-based polysaccharide that allows them to cohere, forming plaque. Sucrose is the only sugar that bacteria can use to form this sticky polysaccharide.[6]
Etymology[edit]
The word sucrose was coined in 1857, by the English chemist William Miller[7] from the French sucre («sugar») and the generic chemical suffix for sugars -ose. The abbreviated term Suc is often used for sucrose in scientific literature.
The name saccharose was coined in 1860 by the French chemist Marcellin Berthelot.[8] Saccharose is an obsolete name for sugars in general, especially sucrose.
Physical and chemical properties[edit]
Structural O-α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside[edit]
In sucrose, the monomers glucose and fructose are linked via an ether bond between C1 on the glucosyl subunit and C2 on the fructosyl unit. The bond is called a glycosidic linkage. Glucose exists predominantly as a mixture of α and β «pyranose» anomers, but sucrose has only the α form. Fructose exists as a mixture of five tautomers but sucrose has only the β-D-fructofuranose form. Unlike most disaccharides, the glycosidic bond in sucrose is formed between the reducing ends of both glucose and fructose, and not between the reducing end of one and the non-reducing end of the other. This linkage inhibits further bonding to other saccharide units, and prevents sucrose from spontaneously reacting with cellular and circulatory macromolecules in the manner that glucose and other reducing sugars do. Since sucrose contains no anomeric hydroxyl groups, it is classified as a non-reducing sugar.
Sucrose crystallizes in the monoclinic space group P21 with room-temperature lattice parameters a = 1.08631 nm, b = 0.87044 nm, c = 0.77624 nm, β = 102.938°.[9][10]
The purity of sucrose is measured by polarimetry, through the rotation of plane-polarized light by a sugar solution. The specific rotation at 20 °C (68 °F) using yellow «sodium-D» light (589 nm) is +66.47°. Commercial samples of sugar are assayed using this parameter. Sucrose does not deteriorate at ambient conditions.
Thermal and oxidative degradation[edit]
Solubility of sucrose in water vs. temperature
| T (°C) | S (g/dL) |
|---|---|
| 50 | 259 |
| 55 | 273 |
| 60 | 289 |
| 65 | 306 |
| 70 | 325 |
| 75 | 346 |
| 80 | 369 |
| 85 | 394 |
| 90 | 420 |
Sucrose does not melt at high temperatures. Instead, it decomposes at 186 °C (367 °F) to form caramel. Like other carbohydrates, it combusts to carbon dioxide and water. Mixing sucrose with the oxidizer potassium nitrate produces the fuel known as rocket candy that is used to propel amateur rocket motors.[11]
C12H22O11 + 6 KNO3 → 9 CO + 3 N2 + 11 H2O + 3 K2CO3
This reaction is somewhat simplified though. Some of the carbon does get fully oxidized to carbon dioxide, and other reactions, such as the water-gas shift reaction also take place. A more accurate theoretical equation is:
C12H22O11 + 6.288 KNO3 → 3.796 CO2 + 5.205 CO + 7.794 H2O + 3.065 H2 + 3.143 N2 + 2.988 K2CO3 + 0.274 KOH[12]
Sucrose burns with chloric acid, formed by the reaction of hydrochloric acid and potassium chlorate:
8 HClO3 + C12H22O11 → 11 H2O + 12 CO2 + 8 HCl
Sucrose can be dehydrated with sulfuric acid to form a black, carbon-rich solid, as indicated in the following idealized equation:
H2SO4 (catalyst) + C12H22O11 → 12 C + 11 H2O + heat (and some H2O + SO3 as a result of the heat).
The formula for sucrose’s decomposition can be represented as a two-step reaction: the first simplified reaction is dehydration of sucrose to pure carbon and water, and then carbon oxidises to CO2 with O2 from air.
C12H22O11 + heat → 12 C + 11 H2O
12C + 12 O2 → 12 CO2
Hydrolysis[edit]
Hydrolysis breaks the glycosidic bond converting sucrose into glucose and fructose. Hydrolysis is, however, so slow that solutions of sucrose can sit for years with negligible change. If the enzyme sucrase is added, however, the reaction will proceed rapidly.[13] Hydrolysis can also be accelerated with acids, such as cream of tartar or lemon juice, both weak acids. Likewise, gastric acidity converts sucrose to glucose and fructose during digestion, the bond between them being an acetal bond which can be broken by an acid.
Given (higher) heats of combustion of 1349.6 kcal/mol for sucrose, 673.0 for glucose, and 675.6 for fructose,[14] hydrolysis releases about 1.0 kcal (4.2 kJ) per mole of sucrose, or about 3 small calories per gram of product.
Synthesis and biosynthesis of sucrose[edit]
The biosynthesis of sucrose proceeds via the precursors UDP-glucose and fructose 6-phosphate, catalyzed by the enzyme sucrose-6-phosphate synthase. The energy for the reaction is gained by the cleavage of uridine diphosphate (UDP).
Sucrose is formed by plants, algae and cyanobacteria but not by other organisms. Sucrose is the end product of photosynthesis and is found naturally in many food plants along with the monosaccharide fructose. In many fruits, such as pineapple and apricot, sucrose is the main sugar. In others, such as grapes and pears, fructose is the main sugar.
Chemical synthesis[edit]
After numerous unsuccessful attempts by others, Raymond Lemieux and George Huber succeeded in synthesizing sucrose from acetylated glucose and fructose in 1953.[15]
Sources[edit]
In nature, sucrose is present in many plants, and in particular their roots, fruits and nectars, because it serves as a way to store energy, primarily from photosynthesis.[16][17] Many mammals, birds, insects and bacteria accumulate and feed on the sucrose in plants and for some it is their main food source. Although honeybees consume sucrose, the honey they produce consists primarily of fructose and glucose, with only trace amounts of sucrose.[18]
As fruits ripen, their sucrose content usually rises sharply, but some fruits contain almost no sucrose at all. This includes grapes, cherries, blueberries, blackberries, figs, pomegranates, tomatoes, avocados, lemons and limes.
Sucrose is a naturally occurring sugar, but with the advent of industrialization, it has been increasingly refined and consumed in all kinds of processed foods.
Production[edit]
History of sucrose refinement[edit]
Table sugar production in the 19th century. Sugar cane plantations (upper image) employed slave or indentured laborers. The picture shows workers harvesting cane, loading it on a boat for transport to the plant, while a European overseer watches in the lower right. The lower image shows a sugar plant with two furnace chimneys. Sugar plants and plantations were harsh, inhumane work.[19]
A sugarloaf was a traditional form for sugar from the 17th to 19th centuries. Sugar nips were required to break off pieces.
The production of table sugar has a long history. Some scholars claim Indians discovered how to crystallize sugar during the Gupta dynasty, around AD 350.[20]
Other scholars point to the ancient manuscripts of China, dated to the 8th century BC, where one of the earliest historical mentions of sugar cane is included along with the fact that their knowledge of sugar cane was derived from India.[21] By about 500 BC, residents of modern-day India began making sugar syrup, cooling it in large flat bowls to produce raw sugar crystals that were easier to store and transport. In the local Indian language, these crystals were called khanda (खण्ड), which is the source of the word candy.[22]
The army of Alexander the Great was halted on the banks of river Indus by the refusal of his troops to go further east. They saw people in the Indian subcontinent growing sugarcane and making «granulated, salt-like sweet powder», locally called sākhar (साखर), pronounced as sakcharon (ζακχαρον) in Greek (Modern Greek, zachari, ζάχαρη). On their return journey, the Greek soldiers carried back some of the «honey-bearing reeds». Sugarcane remained a limited crop for over a millennium. Sugar was a rare commodity and traders of sugar became wealthy. Venice, at the height of its financial power, was the chief sugar-distributing center of Europe.[21] Arabs started producing it in Sicily and Spain. Only after the Crusades did it begin to rival honey as a sweetener in Europe. The Spanish began cultivating sugarcane in the West Indies in 1506 (Cuba in 1523). The Portuguese first cultivated sugarcane in Brazil in 1532.
Sugar remained a luxury in much of the world until the 18th century. Only the wealthy could afford it. In the 18th century, the demand for table sugar boomed in Europe and by the 19th century it had become regarded as a human necessity.[23] The use of sugar grew from use in tea, to cakes, confectionery and chocolates. Suppliers marketed sugar in novel forms, such as solid cones, which required consumers to use a sugar nip, a pliers-like tool, in order to break off pieces.
The demand for cheaper table sugar drove, in part, colonization of tropical islands and nations where labor-intensive sugarcane plantations and table sugar manufacturing could thrive. Growing sugar cane crop in hot humid climates, and producing table sugar in high temperature sugar mills was harsh, inhumane work. The demand for cheap labor for this work, in part, first drove slave trade from Africa (in particular West Africa), followed by indentured labor trade from South Asia (in particular India).[19][24][25] Millions of slaves, followed by millions of indentured laborers were brought into the Caribbean, Indian Ocean, Pacific Islands, East Africa, Natal, north and eastern parts of South America, and southeast Asia. The modern ethnic mix of many nations, settled in the last two centuries, has been influenced by table sugar.[26][27][28]
Beginning in the late 18th century, the production of sugar became increasingly mechanized. The steam engine first powered a sugar mill in Jamaica in 1768, and, soon after, steam replaced direct firing as the source of process heat. During the same century, Europeans began experimenting with sugar production from other crops. Andreas Marggraf identified sucrose in beet root[29] and his student Franz Achard built a sugar beet processing factory in Silesia (Prussia). The beet-sugar industry took off during the Napoleonic Wars, when France and the continent were cut off from Caribbean sugar. In 2009, about 20 percent of the world’s sugar was produced from beets.[30]
Today, a large beet refinery producing around 1,500 tonnes of sugar a day needs a permanent workforce of about 150 for 24-hour production.[citation needed]
Trends[edit]
A table sugar factory in England. The tall diffusers are visible to the middle left where the harvest transforms into a sugar syrup. The boiler and furnace are in the center, where table sugar crystals form. An expressway for transport is visible in the lower left.
Table sugar (sucrose) comes from plant sources. Two important sugar crops predominate: sugarcane (Saccharum spp.) and sugar beets (Beta vulgaris), in which sugar can account for 12% to 20% of the plant’s dry weight. Minor commercial sugar crops include the date palm (Phoenix dactylifera), sorghum (Sorghum vulgare), and the sugar maple (Acer saccharum). Sucrose is obtained by extraction of these crops with hot water; concentration of the extract gives syrups, from which solid sucrose can be crystallized. In 2017, worldwide production of table sugar amounted to 185 million tonnes.[5]
Most cane sugar comes from countries with warm climates, because sugarcane does not tolerate frost. Sugar beets, on the other hand, grow only in cooler temperate regions and do not tolerate extreme heat. About 80 percent of sucrose is derived from sugarcane, the rest almost all from sugar beets.
In mid-2018, India and Brazil had about the same production of sugar – 34 million tonnes – followed by the European Union, Thailand, and China as the major producers.[31] India, the European Union, and China were the leading domestic consumers of sugar in 2018.[31]
Beet sugar comes from regions with cooler climates: northwest and eastern Europe, northern Japan, plus some areas in the United States (including California). In the northern hemisphere, the beet-growing season ends with the start of harvesting around September. Harvesting and processing continues until March in some cases. The availability of processing plant capacity and the weather both influence the duration of harvesting and processing – the industry can store harvested beets until processed, but a frost-damaged beet becomes effectively unprocessable.
The United States sets high sugar prices to support its producers, with the effect that many former purchasers of sugar have switched to corn syrup (beverage manufacturers) or moved out of the country (candy manufacturers).
The low prices of glucose syrups produced from wheat and corn (maize) threaten the traditional sugar market. Used in combination with artificial sweeteners, they can allow drink manufacturers to produce very low-cost goods.
High-fructose corn syrup[edit]
High-fructose corn syrup (HFCS) is significantly cheaper as a sweetener for food and beverage manufacturing than refined sucrose.[32] This has led to sucrose being partially displaced in U.S. industrial food production by HFCS and other non-sucrose natural sweeteners.[32][33]
Reports in public media have regarded HFCS as less safe than sucrose.[32][33] However, the most common forms of HFCS contain either 42 percent fructose, mainly used in processed foods, or 55 percent fructose, mainly used in soft drinks, as compared to sucrose, which is 50 percent fructose. Given approximately equal glucose and fructose content, there does not appear to be a significant difference in safety.[32][34] Clinical dietitians, medical professionals, and the U.S. Food and Drug Administration (FDA) agree that dietary sugars are a source of empty calories associated with certain health problems, and recommend limiting the overall consumption of sugar-based sweeteners.[32][33][34]
Types[edit]
Cane[edit]
Harvested sugarcane from Venezuela ready for processing
Since the 6th century BC, cane sugar producers have crushed the harvested vegetable material from sugarcane in order to collect and filter the juice. They then treat the liquid, often with lime (calcium oxide), to remove impurities and then neutralize it. Boiling the juice then allows the sediment to settle to the bottom for dredging out, while the scum rises to the surface for skimming off. In cooling, the liquid crystallizes, usually in the process of stirring, to produce sugar crystals. Centrifuges usually remove the uncrystallized syrup. The producers can then either sell the sugar product for use as is, or process it further to produce lighter grades. The later processing may take place in another factory in another country.
Sugarcane is a major component of Brazilian agriculture; the country is the world’s largest producer of sugarcane and its derivative products, such as crystallized sugar and ethanol (ethanol fuel).[35]
Beet[edit]
Beet sugar producers slice the washed beets, then extract the sugar with hot water in a «diffuser». An alkaline solution («milk of lime» and carbon dioxide from the lime kiln) then serves to precipitate impurities (see carbonatation). After filtration,[clarification needed] evaporation concentrates the juice to a content of about 70% solids, and controlled crystallisation extracts the sugar. A centrifuge removes the sugar crystals from the liquid, which gets recycled in the crystalliser stages. When economic constraints prevent the removal of more sugar, the manufacturer discards the remaining liquid, now known as molasses, or sells it on to producers of animal feed.
Sieving the resultant white sugar produces different grades for selling.
Cane versus beet[edit]
It is difficult to distinguish between fully refined sugar produced from beet and cane. One way is by isotope analysis of carbon. Cane uses C4 carbon fixation, and beet uses C3 carbon fixation, resulting in a different ratio of 13C and 12C isotopes in the sucrose. Tests are used to detect fraudulent abuse of European Union subsidies or to aid in the detection of adulterated fruit juice.
Sugar cane tolerates hot climates better, but the production of sugar cane needs approximately four times as much water as the production of sugar beet. As a result, some countries that traditionally produced cane sugar (such as Egypt) have built new beet sugar factories since about 2008. Some sugar factories process both sugar cane and sugar beets and extend their processing period in that way.
The production of sugar leaves residues that differ substantially depending on the raw materials used and on the place of production. While cane molasses is often used in food preparation, humans find molasses from sugar beets unpalatable, and it consequently ends up mostly as industrial fermentation feedstock (for example in alcohol distilleries), or as animal feed. Once dried, either type of molasses can serve as fuel for burning.
Pure beet sugar is difficult to find, so labelled, in the marketplace. Although some makers label their product clearly as «pure cane sugar», beet sugar is almost always labeled simply as sugar or pure sugar. Interviews with the 5 major beet sugar-producing companies revealed that many store brands or «private label» sugar products are pure beet sugar. The lot code can be used to identify the company and the plant from which the sugar came, enabling beet sugar to be identified if the codes are known.[36]
Culinary sugars[edit]
Mill white[edit]
Mill white, also called plantation white, crystal sugar or superior sugar is produced from raw sugar. It is exposed to sulfur dioxide during the production to reduce the concentration of color compounds and helps prevent further color development during the crystallization process. Although common to sugarcane-growing areas, this product does not store or ship well. After a few weeks, its impurities tend to promote discoloration and clumping; therefore this type of sugar is generally limited to local consumption.[37]
Blanco directo[edit]
Blanco directo, a white sugar common in India and other south Asian countries, is produced by precipitating many impurities out of cane juice using phosphoric acid and calcium hydroxide, similar to the carbonatation technique used in beet sugar refining. Blanco directo is more pure than mill white sugar, but less pure than white refined.
White refined[edit]
White refined is the most common form of sugar in North America and Europe. Refined sugar is made by dissolving and purifying raw sugar using phosphoric acid similar to the method used for blanco directo, a carbonatation process involving calcium hydroxide and carbon dioxide, or by various filtration strategies. It is then further purified by filtration through a bed of activated carbon or bone char. Beet sugar refineries produce refined white sugar directly without an intermediate raw stage.[clarification needed]
White refined sugar is typically sold as granulated sugar, which has been dried to prevent clumping and comes in various crystal sizes for home and industrial use:
Sugars; clockwise from top left: Refined, unrefined, brown, unprocessed cane
- Coarse-grain, such as sanding sugar (also called «pearl sugar», «decorating sugar», nibbed sugar or sugar nibs) is a coarse grain sugar used to add sparkle and flavor atop baked goods and candies. Its large reflective crystals will not dissolve when subjected to heat.
- Granulated, familiar as table sugar, with a grain size about 0.5 mm across.[38] «Sugar cubes» are lumps for convenient consumption produced by mixing granulated sugar with sugar syrup.
- Caster (0.35 mm),[38] a very fine sugar in Britain and other Commonwealth countries, so-named because the grains are small enough to fit through a sugar caster which is a small vessel with a perforated top, from which to sprinkle sugar at table.[39] Commonly used in baking and mixed drinks, it is sold as «superfine» sugar in the United States. Because of its fineness, it dissolves faster than regular white sugar and is especially useful in meringues and cold liquids. Caster sugar can be prepared at home by grinding granulated sugar for a couple of minutes in a mortar or food processor.
- Powdered, 10X sugar, confectioner’s sugar (0.060 mm), or icing sugar (0.024 mm), produced by grinding sugar to a fine powder. The manufacturer may add a small amount of anticaking agent to prevent clumping — either corn starch (1% to 3%) or tri-calcium phosphate.
Brown sugar comes either from the late stages of cane sugar refining, when sugar forms fine crystals with significant molasses content, or from coating white refined sugar with a cane molasses syrup (blackstrap molasses). Brown sugar’s color and taste becomes stronger with increasing molasses content, as do its moisture-retaining properties. Brown sugars also tend to harden if exposed to the atmosphere, although proper handling can reverse this.
Measurement[edit]
Dissolved sugar content[edit]
Scientists and the sugar industry use degrees Brix (symbol °Bx), introduced by Adolf Brix, as units of measurement of the mass ratio of dissolved substance to water in a liquid. A 25 °Bx sucrose solution has 25 grams of sucrose per 100 grams of liquid; or, to put it another way, 25 grams of sucrose sugar and 75 grams of water exist in the 100 grams of solution.
The Brix degrees are measured using an infrared sensor. This measurement does not equate to Brix degrees from a density or refractive index measurement, because it will specifically measure dissolved sugar concentration instead of all dissolved solids. When using a refractometer, one should report the result as «refractometric dried substance» (RDS). One might speak of a liquid as having 20 °Bx RDS. This refers to a measure of percent by weight of total dried solids and, although not technically the same as Brix degrees determined through an infrared method, renders an accurate measurement of sucrose content, since sucrose in fact forms the majority of dried solids. The advent of in-line infrared Brix measurement sensors has made measuring the amount of dissolved sugar in products economical using a direct measurement.
Consumption[edit]
Refined sugar was a luxury before the 18th century. It became widely popular in the 18th century, then graduated to becoming a necessary food in the 19th century. This evolution of taste and demand for sugar as an essential food ingredient unleashed major economic and social changes.[23] Eventually, table sugar became sufficiently cheap and common enough to influence standard cuisine and flavored drinks.
Sucrose forms a major element in confectionery and desserts. Cooks use it for sweetening. It can also act as a food preservative when used in sufficient concentrations. Sucrose is important to the structure of many foods, including biscuits and cookies, cakes and pies, candy, and ice cream and sorbets. It is a common ingredient in many processed and so-called «junk foods».
Nutritional information[edit]
Sugars, granulated [sucrose]
| Nutritional value per 100 g (3.5 oz) | |
|---|---|
| Energy | 1,620 kJ (390 kcal) |
|
Carbohydrates |
100 g |
|
Fat |
0 g |
|
Protein |
0 g |
| Vitamins | Quantity
%DV† |
| Thiamine (B1) |
0% 0 mg |
| Riboflavin (B2) |
0% 0 mg |
| Niacin (B3) |
0% 0 mg |
| Vitamin C |
0% 0 mg |
| Minerals | Quantity
%DV† |
| Iron |
0% 0 mg |
| Phosphorus |
0% 0 mg |
| Potassium |
0% 2.0 mg |
| Selenium |
1% 0.6 μg |
|
Link to USDA Database entry |
|
|
|
| †Percentages are roughly approximated using US recommendations for adults. Source: USDA FoodData Central |
Fully refined sugar is 99.9% sucrose, thus providing only carbohydrate as dietary nutrient and 390 kilocalories per 100 g serving (USDA data, right table).[40] There are no micronutrients of significance in fully refined sugar (right table).[40]
Metabolism of sucrose[edit]
In humans and other mammals, sucrose is broken down into its constituent monosaccharides, glucose and fructose, by sucrase or isomaltase glycoside hydrolases, which are located in the membrane of the microvilli lining the duodenum.[41][42] The resulting glucose and fructose molecules are then rapidly absorbed into the bloodstream. In bacteria and some animals, sucrose is digested by the enzyme invertase. Sucrose is an easily assimilated macronutrient that provides a quick source of energy, provoking a rapid rise in blood glucose upon ingestion. Sucrose, as a pure carbohydrate, has an energy content of 3.94 kilocalories per gram (or 17 kilojoules per gram).
If consumed excessively, sucrose may contribute to the development of metabolic syndrome, including increased risk for type 2 diabetes, weight gain and obesity in adults and children.[43][44]
Tooth decay[edit]
Tooth decay (dental caries) has become a pronounced health hazard associated with the consumption of sugars, especially sucrose. Oral bacteria such as Streptococcus mutans live in dental plaque and metabolize any free sugars (not just sucrose, but also glucose, lactose, fructose, and cooked starches)[45] into lactic acid. The resultant lactic acid lowers the pH of the tooth’s surface, stripping it of minerals in the process known as tooth decay.[46][47]
All 6-carbon sugars and disaccharides based on 6-carbon sugars can be converted by dental plaque bacteria into acid that demineralizes teeth, but sucrose may be uniquely useful to Streptococcus sanguinis (formerly Streptococcus sanguis) and Streptococcus mutans.[48][49] Sucrose is the only dietary sugar that can be converted to sticky glucans (dextran-like polysaccharides) by extracellular enzymes. These glucans allow the bacteria to adhere to the tooth surface and to build up thick layers of plaque. The anaerobic conditions deep in the plaque encourage the formation of acids, which leads to carious lesions. Thus, sucrose could enable S. mutans, S. sanguinis and many other species of bacteria to adhere strongly and resist natural removal, e.g. by flow of saliva, although they are easily removed by brushing. The glucans and levans (fructose polysaccharides) produced by the plaque bacteria also act as a reserve food supply for the bacteria.
Such a special role of sucrose in the formation of tooth decay is much more significant in light of the almost universal use of sucrose as the most desirable sweetening agent. Widespread replacement of sucrose by high-fructose corn syrup (HFCS) has not diminished the danger from sucrose. If smaller amounts of sucrose are present in the diet, they will still be sufficient for the development of thick, anaerobic plaque and plaque bacteria will metabolise other sugars in the diet,[49] such as the glucose and fructose in HFCS.
Glycemic index[edit]
Sucrose is a disaccharide made up of 50% glucose and 50% fructose and has a glycemic index of 65.[50] Sucrose is digested rapidly,[51][52] but has a relatively low glycemic index due to its content of fructose, which has a minimal effect on blood glucose.[51]
As with other sugars, sucrose is digested into its components via the enzyme sucrase to glucose (blood sugar). The glucose component is transported into the blood where it serves immediate metabolic demands, or is converted and reserved in the liver as glycogen.[52]
Gout[edit]
The occurrence of gout is connected with an excess production of uric acid. A diet rich in sucrose may lead to gout as it raises the level of insulin, which prevents excretion of uric acid from the body. As the concentration of uric acid in the body increases, so does the concentration of uric acid in the joint liquid and beyond a critical concentration, the uric acid begins to precipitate into crystals. Researchers have implicated sugary drinks high in fructose in a surge in cases of gout.[53][54]
Sucrose intolerance[edit]
UN dietary recommendation[edit]
In 2015, the World Health Organization published a new guideline on sugars intake for adults and children, as a result of an extensive review of the available scientific evidence by a multidisciplinary group of experts. The guideline recommends that both adults and children ensure their intake of free sugars (monosaccharides and disaccharides added to foods and beverages by the manufacturer, cook or consumer, and sugars naturally present in honey, syrups, fruit juices and fruit juice concentrates) is less than 10% of total energy intake. A level below 5% of total energy intake brings additional health benefits, especially with regards to dental caries.[55]
Religious concerns[edit]
The sugar refining industry often uses bone char (calcinated animal bones) for decolorizing.[56][57] About 25% of sugar produced in the U.S. is processed using bone char as a filter, the remainder being processed with activated carbon. As bone char does not seem to remain in finished sugar, Jewish religious leaders consider sugar filtered through it to be pareve, meaning that it is neither meat nor dairy and may be used with either type of food. However, the bone char must source to a kosher animal (e.g. cow, sheep) for the sugar to be kosher.[57]
Trade and economics[edit]
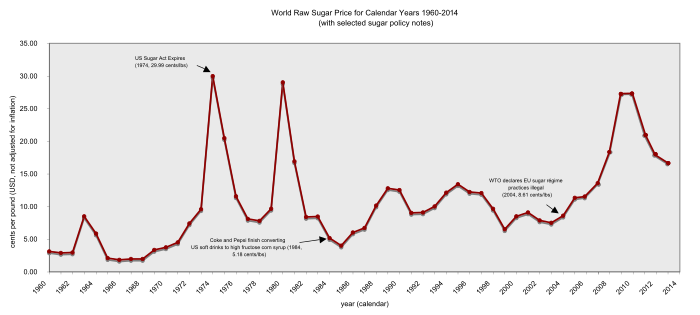
One of the most widely traded commodities in the world throughout history, sugar accounts for around 2% of the global dry cargo market.[citation needed] International sugar prices show great volatility, ranging from around 3 cents to over 60 cents[clarification needed] per pound in the past 50 years. About 100 of the world’s 180 countries produce sugar from beet or cane, a few more refine raw sugar to produce white sugar, and all countries consume sugar. Consumption of sugar ranges from around 3 kilograms (7 lb) per person per annum in Ethiopia to around 40 kg (90 lb) in Belgium.[citation needed] Consumption per capita rises with income per capita until it reaches a plateau of around 35 kg (75 lb) per person per year in middle income countries.
Many countries subsidize sugar production heavily. The European Union, the United States, Japan, and many developing countries subsidize domestic production and maintain high tariffs on imports. Sugar prices in these countries have often up to triple the prices on the international market; today, with world market sugar futures prices currently strong, such prices were typically double world prices.
World raw sugar price 1960–2014
Within international trade bodies, especially in the World Trade Organization (WTO), the «G20» countries led by Brazil have long argued that, because these sugar markets in essence exclude cane sugar imports, the G20 sugar producers receive lower prices than they would under free trade. While both the European Union and United States maintain trade agreements whereby certain developing and least developed countries (LDCs) can sell certain quantities of sugar into their markets, free of the usual import tariffs, countries outside these preferred trade régimes have complained that these arrangements violate the «most favoured nation» principle of international trade. This has led to numerous tariffs and levies in the past.
In 2004, the WTO sided with a group of cane sugar exporting nations (led by Brazil and Australia) and ruled illegal the EU sugar-régime and the accompanying ACP-EU Sugar Protocol, that granted a group of African, Caribbean, and Pacific countries receive preferential access to the European sugar market.[58] In response to this and to other rulings of the WTO, and owing to internal pressures against the EU sugar-régime, the European Commission proposed on 22 June 2005 a radical reform of the EU sugar-régime that cut prices by 39% and eliminated all EU sugar exports.[59]
The African, Caribbean, Pacific and LDC sugar exporters reacted with dismay to the EU sugar proposals.[60] On 25 November 2005, the EC agreed to cut EU sugar prices by 36% as from 2009.
In 2007, it seemed[61] that the U.S. Sugar Program could become the next target for reform. However, some commentators expected heavy lobbying from the U.S. sugar industry, which donated $2.7 million to U.S. House and Senate incumbents in the 2006 U.S. election, more than any other group of U.S. food-growers.[62] Especially prominent among sugar lobbyists were the Fanjul Brothers, so-called «sugar barons» who made the single largest individual contributions of soft money to both the Democratic and Republican parties in the U.S. political system.[63][64]
Small quantities of sugar, especially specialty grades of sugar, reach the market as ‘fair trade’ commodities; the fair trade system produces and sells these products with the understanding that a larger-than-usual fraction of the revenue will support small farmers in the developing world. However, whilst the Fairtrade Foundation offers a premium of $60.00 per tonne to small farmers for sugar branded as «Fairtrade»,[65] government schemes such as the U.S. Sugar Program and the ACP-EU Sugar Protocol offer premiums of around $400.00 per tonne above world market prices. However, the EU announced on 14 September 2007 that it had offered «to eliminate all duties and quotas on the import of sugar into the EU».[66]
The U.S. Sugar Association subsequently launched a campaign to promote sugar over artificial substitutes. The Association now aggressively contradicts many common beliefs regarding negative side-effects of sugar consumption. The campaign aired a high-profile television commercial during the 2007 Primetime Emmy Awards on FOX Television.
References[edit]
- ^ Sucrose, International Chemical Safety Card 1507, Geneva: International Programme on Chemical Safety, November 2003
- ^ CRC Handbook of Chemistry and Physics, 49th edition, 1968–1969, p. D-188.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. «#0574». National Institute for Occupational Safety and Health (NIOSH).
- ^ «57-50-1 — CZMRCDWAGMRECN-UGDNZRGBSA-N — Sucrose [JAN:NF]». ChemIDplus. Archived from the original on 2014-08-12. Retrieved 2014-08-10.
- ^ a b Marcy Nicholson (17 November 2017). «World 2017/18 sugar production, consumption seen at record: USDA». Reuters. Archived from the original on 14 September 2019. Retrieved 21 December 2019.
- ^ Ryan KJ, Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 978-0-8385-8529-0.
- ^ William Allen Miller, Elements of Chemistry: Theoretical and Practical, Part III. Organic Chemistry (London, England: John W. Parker and son, 1857), pages 52 and 54 Archived 2017-04-24 at the Wayback Machine.
- ^ Marcellin Berthelot, Chimie organique fondée sur la synthèse (Paris, France: Mallet-Bachelier, 1860), pp. 254–55 Archived 2014-06-27 at the Wayback Machine.
- ^ Beevers, C. A.; McDonald, T. R. R.; Robertson, J. H.; Stern, F. (1952). «The crystal structure of sucrose». Acta Crystallogr. 5 (5): 689–90. doi:10.1107/S0365110X52001908.
- ^ Hynes, R. C.; Le Page, Y. (1991). «Sucrose, a convenient test crystal for absolute structures». Journal of Applied Crystallography. 24 (4): 352. doi:10.1107/S0021889891002492.
- ^ Nakka, Richard. «Potassium Nitrate/Sucrose Propellant (KNSU)». Experimental Rocketry. Archived from the original on 2015-10-26. Retrieved 2015-11-19.
- ^ Nakka, Richard. «KN-Sucrose (KNSU) Propellant Chemistry and Performance Characteristics». Experimental Rocketry. Archived from the original on 2014-11-18. Retrieved 2014-08-21.
- ^ «Sucrase» Archived 2010-04-25 at the Wayback Machine, Encyclopædia Britannica Online
- ^ All three from CRC Handbook of Chemistry and Physics, 49th edition, 1968-1969, pp. D-184-189.
- ^ Lemieux, R. U.; Huber, G. (1953). «A chemical synthesis of sucrose». J. Am. Chem. Soc. 75 (16): 4118. doi:10.1021/ja01112a545.
- ^ John E. Lunn (December 2008). «Sucrose Metabolism». Encyclopedia of Life Sciences. eLS. John Wiley & Sons Ltd. doi:10.1002/9780470015902.a0021259. ISBN 978-0470016176.
- ^ «Foods highest in Sucrose». SelfNutritiondata. Condé Nast. Archived from the original on 2015-07-19.
- ^ Douglas M. Considine (1982). Considine, Douglas M; Considine, Glenn D (eds.). Foods and Food Production Encyclopedia (1 ed.). Van Nostrand Reinhold Company Inc. p. 956. doi:10.1007/978-1-4684-8511-0. ISBN 978-1-4684-8513-4.
- ^ a b «Forced Labour». The National Archives, Government of the United Kingdom. 2010. Archived from the original on 2016-12-04.
- ^
Adas, Michael (2001). Agricultural and Pastoral Societies in Ancient and Classical History Archived 2013-06-14 at the Wayback Machine. Temple University Press. ISBN 1-56639-832-0. p. 311. - ^ a b Rolph, George (1873). Something about sugar: its history, growth, manufacture and distribution. San Francisco, J. J. Newbegin.
- ^ «Sugarcane: Saccharum Offcinarum» (PDF). USAID, Govt of United States. 2006. p. 7.1. Archived from the original (PDF) on 2013-11-06.
- ^ a b Mintz, Sidney (1986). Sweetness and Power: The Place of Sugar in Modern History. Penguin. ISBN 978-0-14-009233-2.
- ^ Lai, Walton (1993). Indentured labor, Caribbean sugar: Chinese and Indian migrants to the British West Indies, 1838–1918. ISBN 978-0-8018-7746-9.
- ^ Vertovik, Steven (1995). Cohen, Robin (ed.). The Cambridge survey of world migration. pp. 57–68. ISBN 978-0-521-44405-7.
- ^ Laurence, K (1994). A Question of Labour: Indentured Immigration Into Trinidad & British Guiana, 1875–1917. St Martin’s Press. ISBN 978-0-312-12172-3.
- ^ «St. Lucia’s Indian Arrival Day». Caribbean Repeating Islands. 2009-05-07. Archived from the original on 2017-04-24.
- ^ «Indian indentured labourers». The National Archives, Government of the United Kingdom. 2010. Archived from the original on 2011-12-12.
- ^ Marggraf (1747) «Experiences chimiques faites dans le dessein de tirer un veritable sucre de diverses plantes, qui croissent dans nos contrées» Archived 2016-06-24 at the Wayback Machine [Chemical experiments made with the intention of extracting real sugar from diverse plants that grow in our lands], Histoire de l’académie royale des sciences et belles-lettres de Berlin, pp. 79–90.
- ^ «Agribusiness Handbook: Sugar beet white sugar» (PDF). Food and Agriculture Organization, United Nations. 2009. Archived (PDF) from the original on 2015-09-05.
- ^ a b «Sugar: World Markets and Trade» (PDF). Office of Global Analysis, Foreign Agricultural Service, US Department of Agriculture. 4 November 2018. Archived (PDF) from the original on 21 October 2021. Retrieved 21 December 2018.
- ^ a b c d e Webb, Densie (7 June 2017). «Is High-Fructose Corn Syrup Worse Than Regular Sugar?». Berkeley Wellness, University of California — Berkeley. Archived from the original on 6 August 2019. Retrieved 19 September 2019.
- ^ a b c Parker-Pope, Tara (20 September 2010). «In Worries About Sweeteners, Think of All Sugars». New York Times. Archived from the original on 12 June 2018. Retrieved 2 January 2018.
- ^ a b «High Fructose Corn Syrup: Questions and Answers». US Food and Drug Administration. 5 November 2014. Archived from the original on 25 January 2018. Retrieved 2 January 2018.
- ^ «Top Sugarcane Producing Countries: Brazil outperforms its next 6 closest competitors combined». World Atlas. 25 April 2017. Archived from the original on 3 January 2018. Retrieved 2 January 2018.
- ^ January 2010 Newsletter Archived 2010-09-24 at the Wayback Machine, IBS Treatment Center
- ^ Steindl, Roderick (2005). Hogarth, DM (ed.). Syrup Clarification for Plantation White Sugar to meet New Quality Standards (PDF). Proceedings of the XXV Congress of International Society of Sugar Cane Technologists. Guatemala, Guatemala City. pp. 106–16. Archived (PDF) from the original on 2013-08-10.
- ^ a b Sugar Crystal Challenge Archived 2013-05-08 at the Wayback Machine. IEEE
- ^ «castor, n.2.» OED Online. Oxford University Press, June 2017. Web. 25 July 2017. It says castor is a misspelling that is now the preferred spelling.
- ^ a b «Nutrition Facts for sugars, granulated [sucrose] per 100 g (USDA National Nutrient Database, SR-21)». Conde Nast. 2014. Archived from the original on 7 March 2015. Retrieved 6 March 2015.
- ^ Gray GM (1971). «Intestinal digestion and maldigestion of dietary carbohydrate». Annual Review of Medicine. 22: 391–404. doi:10.1146/annurev.me.22.020171.002135. PMID 4944426.
- ^ Kaneko J.J. (2008) «Carbohydrate metabolism and its diseases» Archived 2014-09-22 at the Wayback Machine, p. 46 in Kaneko J.J., Harvey J.W., Bruss M.L. (eds.) Clinical Biochemistry of Domestic Animals, San Diego, CA: Academic Press, ISBN 012370491X.
- ^ Malik, V. S.; Popkin, B. M.; Bray, G. A.; Despres, J.-P.; Willett, W. C.; Hu, F. B. (2010). «Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis». Diabetes Care. 33 (11): 2477–83. doi:10.2337/dc10-1079. PMC 2963518. PMID 20693348.
- ^ Malik, Vasanti S.; Pan, An; Willett, Walter C.; Hu, Frank B. (2013-10-01). «Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis». The American Journal of Clinical Nutrition. 98 (4): 1084–1102. doi:10.3945/ajcn.113.058362. ISSN 0002-9165. PMC 3778861. PMID 23966427. Archived from the original on 2018-01-09. Retrieved 2018-12-21.
- ^ «What causes tooth decay?». Animated-teeth.com. Archived from the original on 2010-02-09. Retrieved 2010-05-05.
- ^ Tooth Decay Archived 2014-10-25 at the Wayback Machine. Elmhurst.edu. Retrieved on 2011-11-18.
- ^ What causes tooth decay? Archived 2010-02-09 at the Wayback Machine. Animated-teeth.com. Retrieved on 2011-11-18.
- ^ Tanzer, JM (August 1979). «Essential dependence of smooth surface caries on, and augmentation of fissure caries by, sucrose and Streptococcus mutans infection». Infection and Immunity. 25 (2): 526–31. doi:10.1128/IAI.25.2.526-531.1979. PMC 443577. PMID 489122.
- ^ a b Darlington, W. (August 1979). Metabolism of sucrose by Stepococcus sanguis 804 (NCTC 10904) and its relevance to the oral environment (Ph.D Thesis). University of Glasgow.
- ^ Wolever, Thomas M. S. (2006). The Glycaemic Index: A Physiological Classification of Dietary Carbohydrate. CABI. p. 64. ISBN 9781845930523. Archived from the original on 2017-12-16.
- ^ a b Wolever, Thomas M. S. (2006). The Glycaemic Index: A Physiological Classification of Dietary Carbohydrate. CABI. p. 65. ISBN 9781845930523. Archived from the original on 2017-12-16.
- ^ a b Food and Nutrition Board, Institute of Medicine of the US National Academies (2005). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). National Academies Press. p. 323. doi:10.17226/10490. ISBN 978-0-309-08525-0. Archived from the original on 2015-07-15.
- ^ Gout surge blamed on sweet drinks Archived 2009-08-16 at the Wayback Machine, BBC News, 1 February 2008
- ^ Magidenko, Leonid (2007-07-30). «Nutrients for Gout – good and bad». ABCVitaminsLife.com. Archived from the original on 2009-02-23. Retrieved 2010-05-05.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ^ See Guideline: Sugars intake for adults and children. Geneva: World Health Organization; 2015 Archived 2015-08-17 at the Wayback Machine
- ^ The Great Sugar Debate: Is it Vegan? Archived 2009-09-19 at the Wayback Machine. Vegfamily.com. Retrieved on 2011-11-18.
- ^ a b Yacoubou, MS, Jeanne (2007). «Is Your Sugar Vegan? An Update on Sugar Processing Practices» (PDF). Vegetarian Journal. Baltimore, MD: The Vegetarian Resource Group. 26 (4): 16–20. Archived (PDF) from the original on 2008-04-09. Retrieved 2007-04-04.
- ^ EC export subsidies on sugar (PDF). wto.org (Report). World Trade Organization. Archived from the original (PDF) on 2009-04-10. Retrieved 2011-11-18.
- ^ «Sugar». ec.europa.eu. Agriculture. European Commission. 2004-07-14. Archived from the original on 2009-08-22. Retrieved 2011-11-18.
- ^ The Fiji Communiqué on Sugar. acpsec.org (Report). ACP Group of States. 2007-05-03. Archived from the original on 2011-07-24. Retrieved 2011-11-18.
- ^ «International Sugar Trade Coalition». sugarcoalition.org (main page). Archived from the original on 2009-06-01. Retrieved 2011-11-18.
- ^ «Seeing sugar’s future in fuel». Business. The New York Times. 8 October 2007. Archived from the original on 2017-07-07.
- ^ «America’s sugar daddies». The New York Times. 11 November 2003. Archived from the original on 2016-03-07.
- ^ «Sugar daddie$». Mother Jones. 1997-05-01. Archived from the original on 2008-12-02. Retrieved 2010-05-05.
- ^ «Sugar». fairtrade.net. Fairtrade Labelling Organizations International (FLO). Archived from the original on 2012-08-02.
- ^ Trade Issues. External Trade. Ec.europa.eu (Report). European Commission. 2010-05-06. Archived from the original on 2009-09-05. Retrieved 2011-11-18.
Further reading[edit]
- Yudkin, J.; Edelman, J.; Hough, L. (1973). Sugar: Chemical, Biological and Nutritional Aspects of Sucrose. Butterworth. ISBN 978-0-408-70172-3.
External links[edit]
Wikimedia Commons has media related to Sucrose.
- 3D images of sucrose
- CDC – NIOSH Pocket Guide to Chemical Hazards
Sucrose

|
|

|
|

|
|
| Names | |
|---|---|
| IUPAC name
β-D-Fructofuranosyl α-D-glucopyranoside |
|
| Preferred IUPAC name
(2R,3R,4S,5S,6R)-2-{[(2S,3S,4S,5R)-3,4-Dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|
Other names
|
|
| Identifiers | |
|
CAS Number |
|
|
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.000.304 |
| EC Number |
|
|
IUPHAR/BPS |
|
| KEGG |
|
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
|
CompTox Dashboard (EPA) |
|
|
InChI
|
|
|
SMILES
|
|
| Properties[1] | |
|
Chemical formula |
C 12H 22O 11 |
| Molar mass | 342.30 g/mol |
| Appearance | white solid |
| Density | 1.587 g/cm3 (0.0573 lb/cu in), solid |
| Melting point | None; decomposes at 186°C (367°F; 459 K) |
|
Solubility in water |
~200 g/dL (25 °C (77 °F)) |
| log P | −3.76 |
| Structure | |
|
Crystal structure |
Monoclinic |
|
Space group |
P21 |
| Thermochemistry | |
|
Std enthalpy of |
1,349.6 kcal/mol (5,647 kJ/mol)[2] (Higher heating value) |
| Hazards | |
| NFPA 704 (fire diamond) |
0 1 0 |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose) |
29700 mg/kg (oral, rat)[4] |
| NIOSH (US health exposure limits): | |
|
PEL (Permissible) |
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[3] |
|
REL (Recommended) |
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[3] |
|
IDLH (Immediate danger) |
N.D.[3] |
| Safety data sheet (SDS) | ICSC 1507 |
| Related compounds | |
|
Related compounds |
Lactose Maltose |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula C
12H
22O
11.
For human consumption, sucrose is extracted and refined from either sugarcane or sugar beet. Sugar mills – typically located in tropical regions near where sugarcane is grown – crush the cane and produce raw sugar which is shipped to other factories for refining into pure sucrose. Sugar beet factories are located in temperate climates where the beet is grown, and process the beets directly into refined sugar. The sugar-refining process involves washing the raw sugar crystals before dissolving them into a sugar syrup which is filtered and then passed over carbon to remove any residual colour. The sugar syrup is then concentrated by boiling under a vacuum and crystallized as the final purification process to produce crystals of pure sucrose that are clear, odorless, and sweet.
Sugar is often an added ingredient in food production and recipes. About 185 million tonnes of sugar were produced worldwide in 2017.[5]
Sucrose is particularly dangerous as a risk factor for tooth decay because Streptococcus mutans bacteria convert it into a sticky, extracellular, dextran-based polysaccharide that allows them to cohere, forming plaque. Sucrose is the only sugar that bacteria can use to form this sticky polysaccharide.[6]
Etymology[edit]
The word sucrose was coined in 1857, by the English chemist William Miller[7] from the French sucre («sugar») and the generic chemical suffix for sugars -ose. The abbreviated term Suc is often used for sucrose in scientific literature.
The name saccharose was coined in 1860 by the French chemist Marcellin Berthelot.[8] Saccharose is an obsolete name for sugars in general, especially sucrose.
Physical and chemical properties[edit]
Structural O-α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside[edit]
In sucrose, the monomers glucose and fructose are linked via an ether bond between C1 on the glucosyl subunit and C2 on the fructosyl unit. The bond is called a glycosidic linkage. Glucose exists predominantly as a mixture of α and β «pyranose» anomers, but sucrose has only the α form. Fructose exists as a mixture of five tautomers but sucrose has only the β-D-fructofuranose form. Unlike most disaccharides, the glycosidic bond in sucrose is formed between the reducing ends of both glucose and fructose, and not between the reducing end of one and the non-reducing end of the other. This linkage inhibits further bonding to other saccharide units, and prevents sucrose from spontaneously reacting with cellular and circulatory macromolecules in the manner that glucose and other reducing sugars do. Since sucrose contains no anomeric hydroxyl groups, it is classified as a non-reducing sugar.
Sucrose crystallizes in the monoclinic space group P21 with room-temperature lattice parameters a = 1.08631 nm, b = 0.87044 nm, c = 0.77624 nm, β = 102.938°.[9][10]
The purity of sucrose is measured by polarimetry, through the rotation of plane-polarized light by a sugar solution. The specific rotation at 20 °C (68 °F) using yellow «sodium-D» light (589 nm) is +66.47°. Commercial samples of sugar are assayed using this parameter. Sucrose does not deteriorate at ambient conditions.
Thermal and oxidative degradation[edit]
Solubility of sucrose in water vs. temperature
| T (°C) | S (g/dL) |
|---|---|
| 50 | 259 |
| 55 | 273 |
| 60 | 289 |
| 65 | 306 |
| 70 | 325 |
| 75 | 346 |
| 80 | 369 |
| 85 | 394 |
| 90 | 420 |
Sucrose does not melt at high temperatures. Instead, it decomposes at 186 °C (367 °F) to form caramel. Like other carbohydrates, it combusts to carbon dioxide and water. Mixing sucrose with the oxidizer potassium nitrate produces the fuel known as rocket candy that is used to propel amateur rocket motors.[11]
C12H22O11 + 6 KNO3 → 9 CO + 3 N2 + 11 H2O + 3 K2CO3
This reaction is somewhat simplified though. Some of the carbon does get fully oxidized to carbon dioxide, and other reactions, such as the water-gas shift reaction also take place. A more accurate theoretical equation is:
C12H22O11 + 6.288 KNO3 → 3.796 CO2 + 5.205 CO + 7.794 H2O + 3.065 H2 + 3.143 N2 + 2.988 K2CO3 + 0.274 KOH[12]
Sucrose burns with chloric acid, formed by the reaction of hydrochloric acid and potassium chlorate:
8 HClO3 + C12H22O11 → 11 H2O + 12 CO2 + 8 HCl
Sucrose can be dehydrated with sulfuric acid to form a black, carbon-rich solid, as indicated in the following idealized equation:
H2SO4 (catalyst) + C12H22O11 → 12 C + 11 H2O + heat (and some H2O + SO3 as a result of the heat).
The formula for sucrose’s decomposition can be represented as a two-step reaction: the first simplified reaction is dehydration of sucrose to pure carbon and water, and then carbon oxidises to CO2 with O2 from air.
C12H22O11 + heat → 12 C + 11 H2O
12C + 12 O2 → 12 CO2
Hydrolysis[edit]
Hydrolysis breaks the glycosidic bond converting sucrose into glucose and fructose. Hydrolysis is, however, so slow that solutions of sucrose can sit for years with negligible change. If the enzyme sucrase is added, however, the reaction will proceed rapidly.[13] Hydrolysis can also be accelerated with acids, such as cream of tartar or lemon juice, both weak acids. Likewise, gastric acidity converts sucrose to glucose and fructose during digestion, the bond between them being an acetal bond which can be broken by an acid.
Given (higher) heats of combustion of 1349.6 kcal/mol for sucrose, 673.0 for glucose, and 675.6 for fructose,[14] hydrolysis releases about 1.0 kcal (4.2 kJ) per mole of sucrose, or about 3 small calories per gram of product.
Synthesis and biosynthesis of sucrose[edit]
The biosynthesis of sucrose proceeds via the precursors UDP-glucose and fructose 6-phosphate, catalyzed by the enzyme sucrose-6-phosphate synthase. The energy for the reaction is gained by the cleavage of uridine diphosphate (UDP).
Sucrose is formed by plants, algae and cyanobacteria but not by other organisms. Sucrose is the end product of photosynthesis and is found naturally in many food plants along with the monosaccharide fructose. In many fruits, such as pineapple and apricot, sucrose is the main sugar. In others, such as grapes and pears, fructose is the main sugar.
Chemical synthesis[edit]
After numerous unsuccessful attempts by others, Raymond Lemieux and George Huber succeeded in synthesizing sucrose from acetylated glucose and fructose in 1953.[15]
Sources[edit]
In nature, sucrose is present in many plants, and in particular their roots, fruits and nectars, because it serves as a way to store energy, primarily from photosynthesis.[16][17] Many mammals, birds, insects and bacteria accumulate and feed on the sucrose in plants and for some it is their main food source. Although honeybees consume sucrose, the honey they produce consists primarily of fructose and glucose, with only trace amounts of sucrose.[18]
As fruits ripen, their sucrose content usually rises sharply, but some fruits contain almost no sucrose at all. This includes grapes, cherries, blueberries, blackberries, figs, pomegranates, tomatoes, avocados, lemons and limes.
Sucrose is a naturally occurring sugar, but with the advent of industrialization, it has been increasingly refined and consumed in all kinds of processed foods.
Production[edit]
History of sucrose refinement[edit]
Table sugar production in the 19th century. Sugar cane plantations (upper image) employed slave or indentured laborers. The picture shows workers harvesting cane, loading it on a boat for transport to the plant, while a European overseer watches in the lower right. The lower image shows a sugar plant with two furnace chimneys. Sugar plants and plantations were harsh, inhumane work.[19]
A sugarloaf was a traditional form for sugar from the 17th to 19th centuries. Sugar nips were required to break off pieces.
The production of table sugar has a long history. Some scholars claim Indians discovered how to crystallize sugar during the Gupta dynasty, around AD 350.[20]
Other scholars point to the ancient manuscripts of China, dated to the 8th century BC, where one of the earliest historical mentions of sugar cane is included along with the fact that their knowledge of sugar cane was derived from India.[21] By about 500 BC, residents of modern-day India began making sugar syrup, cooling it in large flat bowls to produce raw sugar crystals that were easier to store and transport. In the local Indian language, these crystals were called khanda (खण्ड), which is the source of the word candy.[22]
The army of Alexander the Great was halted on the banks of river Indus by the refusal of his troops to go further east. They saw people in the Indian subcontinent growing sugarcane and making «granulated, salt-like sweet powder», locally called sākhar (साखर), pronounced as sakcharon (ζακχαρον) in Greek (Modern Greek, zachari, ζάχαρη). On their return journey, the Greek soldiers carried back some of the «honey-bearing reeds». Sugarcane remained a limited crop for over a millennium. Sugar was a rare commodity and traders of sugar became wealthy. Venice, at the height of its financial power, was the chief sugar-distributing center of Europe.[21] Arabs started producing it in Sicily and Spain. Only after the Crusades did it begin to rival honey as a sweetener in Europe. The Spanish began cultivating sugarcane in the West Indies in 1506 (Cuba in 1523). The Portuguese first cultivated sugarcane in Brazil in 1532.
Sugar remained a luxury in much of the world until the 18th century. Only the wealthy could afford it. In the 18th century, the demand for table sugar boomed in Europe and by the 19th century it had become regarded as a human necessity.[23] The use of sugar grew from use in tea, to cakes, confectionery and chocolates. Suppliers marketed sugar in novel forms, such as solid cones, which required consumers to use a sugar nip, a pliers-like tool, in order to break off pieces.
The demand for cheaper table sugar drove, in part, colonization of tropical islands and nations where labor-intensive sugarcane plantations and table sugar manufacturing could thrive. Growing sugar cane crop in hot humid climates, and producing table sugar in high temperature sugar mills was harsh, inhumane work. The demand for cheap labor for this work, in part, first drove slave trade from Africa (in particular West Africa), followed by indentured labor trade from South Asia (in particular India).[19][24][25] Millions of slaves, followed by millions of indentured laborers were brought into the Caribbean, Indian Ocean, Pacific Islands, East Africa, Natal, north and eastern parts of South America, and southeast Asia. The modern ethnic mix of many nations, settled in the last two centuries, has been influenced by table sugar.[26][27][28]
Beginning in the late 18th century, the production of sugar became increasingly mechanized. The steam engine first powered a sugar mill in Jamaica in 1768, and, soon after, steam replaced direct firing as the source of process heat. During the same century, Europeans began experimenting with sugar production from other crops. Andreas Marggraf identified sucrose in beet root[29] and his student Franz Achard built a sugar beet processing factory in Silesia (Prussia). The beet-sugar industry took off during the Napoleonic Wars, when France and the continent were cut off from Caribbean sugar. In 2009, about 20 percent of the world’s sugar was produced from beets.[30]
Today, a large beet refinery producing around 1,500 tonnes of sugar a day needs a permanent workforce of about 150 for 24-hour production.[citation needed]
Trends[edit]
A table sugar factory in England. The tall diffusers are visible to the middle left where the harvest transforms into a sugar syrup. The boiler and furnace are in the center, where table sugar crystals form. An expressway for transport is visible in the lower left.
Table sugar (sucrose) comes from plant sources. Two important sugar crops predominate: sugarcane (Saccharum spp.) and sugar beets (Beta vulgaris), in which sugar can account for 12% to 20% of the plant’s dry weight. Minor commercial sugar crops include the date palm (Phoenix dactylifera), sorghum (Sorghum vulgare), and the sugar maple (Acer saccharum). Sucrose is obtained by extraction of these crops with hot water; concentration of the extract gives syrups, from which solid sucrose can be crystallized. In 2017, worldwide production of table sugar amounted to 185 million tonnes.[5]
Most cane sugar comes from countries with warm climates, because sugarcane does not tolerate frost. Sugar beets, on the other hand, grow only in cooler temperate regions and do not tolerate extreme heat. About 80 percent of sucrose is derived from sugarcane, the rest almost all from sugar beets.
In mid-2018, India and Brazil had about the same production of sugar – 34 million tonnes – followed by the European Union, Thailand, and China as the major producers.[31] India, the European Union, and China were the leading domestic consumers of sugar in 2018.[31]
Beet sugar comes from regions with cooler climates: northwest and eastern Europe, northern Japan, plus some areas in the United States (including California). In the northern hemisphere, the beet-growing season ends with the start of harvesting around September. Harvesting and processing continues until March in some cases. The availability of processing plant capacity and the weather both influence the duration of harvesting and processing – the industry can store harvested beets until processed, but a frost-damaged beet becomes effectively unprocessable.
The United States sets high sugar prices to support its producers, with the effect that many former purchasers of sugar have switched to corn syrup (beverage manufacturers) or moved out of the country (candy manufacturers).
The low prices of glucose syrups produced from wheat and corn (maize) threaten the traditional sugar market. Used in combination with artificial sweeteners, they can allow drink manufacturers to produce very low-cost goods.
High-fructose corn syrup[edit]
High-fructose corn syrup (HFCS) is significantly cheaper as a sweetener for food and beverage manufacturing than refined sucrose.[32] This has led to sucrose being partially displaced in U.S. industrial food production by HFCS and other non-sucrose natural sweeteners.[32][33]
Reports in public media have regarded HFCS as less safe than sucrose.[32][33] However, the most common forms of HFCS contain either 42 percent fructose, mainly used in processed foods, or 55 percent fructose, mainly used in soft drinks, as compared to sucrose, which is 50 percent fructose. Given approximately equal glucose and fructose content, there does not appear to be a significant difference in safety.[32][34] Clinical dietitians, medical professionals, and the U.S. Food and Drug Administration (FDA) agree that dietary sugars are a source of empty calories associated with certain health problems, and recommend limiting the overall consumption of sugar-based sweeteners.[32][33][34]
Types[edit]
Cane[edit]
Harvested sugarcane from Venezuela ready for processing
Since the 6th century BC, cane sugar producers have crushed the harvested vegetable material from sugarcane in order to collect and filter the juice. They then treat the liquid, often with lime (calcium oxide), to remove impurities and then neutralize it. Boiling the juice then allows the sediment to settle to the bottom for dredging out, while the scum rises to the surface for skimming off. In cooling, the liquid crystallizes, usually in the process of stirring, to produce sugar crystals. Centrifuges usually remove the uncrystallized syrup. The producers can then either sell the sugar product for use as is, or process it further to produce lighter grades. The later processing may take place in another factory in another country.
Sugarcane is a major component of Brazilian agriculture; the country is the world’s largest producer of sugarcane and its derivative products, such as crystallized sugar and ethanol (ethanol fuel).[35]
Beet[edit]
Beet sugar producers slice the washed beets, then extract the sugar with hot water in a «diffuser». An alkaline solution («milk of lime» and carbon dioxide from the lime kiln) then serves to precipitate impurities (see carbonatation). After filtration,[clarification needed] evaporation concentrates the juice to a content of about 70% solids, and controlled crystallisation extracts the sugar. A centrifuge removes the sugar crystals from the liquid, which gets recycled in the crystalliser stages. When economic constraints prevent the removal of more sugar, the manufacturer discards the remaining liquid, now known as molasses, or sells it on to producers of animal feed.
Sieving the resultant white sugar produces different grades for selling.
Cane versus beet[edit]
It is difficult to distinguish between fully refined sugar produced from beet and cane. One way is by isotope analysis of carbon. Cane uses C4 carbon fixation, and beet uses C3 carbon fixation, resulting in a different ratio of 13C and 12C isotopes in the sucrose. Tests are used to detect fraudulent abuse of European Union subsidies or to aid in the detection of adulterated fruit juice.
Sugar cane tolerates hot climates better, but the production of sugar cane needs approximately four times as much water as the production of sugar beet. As a result, some countries that traditionally produced cane sugar (such as Egypt) have built new beet sugar factories since about 2008. Some sugar factories process both sugar cane and sugar beets and extend their processing period in that way.
The production of sugar leaves residues that differ substantially depending on the raw materials used and on the place of production. While cane molasses is often used in food preparation, humans find molasses from sugar beets unpalatable, and it consequently ends up mostly as industrial fermentation feedstock (for example in alcohol distilleries), or as animal feed. Once dried, either type of molasses can serve as fuel for burning.
Pure beet sugar is difficult to find, so labelled, in the marketplace. Although some makers label their product clearly as «pure cane sugar», beet sugar is almost always labeled simply as sugar or pure sugar. Interviews with the 5 major beet sugar-producing companies revealed that many store brands or «private label» sugar products are pure beet sugar. The lot code can be used to identify the company and the plant from which the sugar came, enabling beet sugar to be identified if the codes are known.[36]
Culinary sugars[edit]
Mill white[edit]
Mill white, also called plantation white, crystal sugar or superior sugar is produced from raw sugar. It is exposed to sulfur dioxide during the production to reduce the concentration of color compounds and helps prevent further color development during the crystallization process. Although common to sugarcane-growing areas, this product does not store or ship well. After a few weeks, its impurities tend to promote discoloration and clumping; therefore this type of sugar is generally limited to local consumption.[37]
Blanco directo[edit]
Blanco directo, a white sugar common in India and other south Asian countries, is produced by precipitating many impurities out of cane juice using phosphoric acid and calcium hydroxide, similar to the carbonatation technique used in beet sugar refining. Blanco directo is more pure than mill white sugar, but less pure than white refined.
White refined[edit]
White refined is the most common form of sugar in North America and Europe. Refined sugar is made by dissolving and purifying raw sugar using phosphoric acid similar to the method used for blanco directo, a carbonatation process involving calcium hydroxide and carbon dioxide, or by various filtration strategies. It is then further purified by filtration through a bed of activated carbon or bone char. Beet sugar refineries produce refined white sugar directly without an intermediate raw stage.[clarification needed]
White refined sugar is typically sold as granulated sugar, which has been dried to prevent clumping and comes in various crystal sizes for home and industrial use:
Sugars; clockwise from top left: Refined, unrefined, brown, unprocessed cane
- Coarse-grain, such as sanding sugar (also called «pearl sugar», «decorating sugar», nibbed sugar or sugar nibs) is a coarse grain sugar used to add sparkle and flavor atop baked goods and candies. Its large reflective crystals will not dissolve when subjected to heat.
- Granulated, familiar as table sugar, with a grain size about 0.5 mm across.[38] «Sugar cubes» are lumps for convenient consumption produced by mixing granulated sugar with sugar syrup.
- Caster (0.35 mm),[38] a very fine sugar in Britain and other Commonwealth countries, so-named because the grains are small enough to fit through a sugar caster which is a small vessel with a perforated top, from which to sprinkle sugar at table.[39] Commonly used in baking and mixed drinks, it is sold as «superfine» sugar in the United States. Because of its fineness, it dissolves faster than regular white sugar and is especially useful in meringues and cold liquids. Caster sugar can be prepared at home by grinding granulated sugar for a couple of minutes in a mortar or food processor.
- Powdered, 10X sugar, confectioner’s sugar (0.060 mm), or icing sugar (0.024 mm), produced by grinding sugar to a fine powder. The manufacturer may add a small amount of anticaking agent to prevent clumping — either corn starch (1% to 3%) or tri-calcium phosphate.
Brown sugar comes either from the late stages of cane sugar refining, when sugar forms fine crystals with significant molasses content, or from coating white refined sugar with a cane molasses syrup (blackstrap molasses). Brown sugar’s color and taste becomes stronger with increasing molasses content, as do its moisture-retaining properties. Brown sugars also tend to harden if exposed to the atmosphere, although proper handling can reverse this.
Measurement[edit]
Dissolved sugar content[edit]
Scientists and the sugar industry use degrees Brix (symbol °Bx), introduced by Adolf Brix, as units of measurement of the mass ratio of dissolved substance to water in a liquid. A 25 °Bx sucrose solution has 25 grams of sucrose per 100 grams of liquid; or, to put it another way, 25 grams of sucrose sugar and 75 grams of water exist in the 100 grams of solution.
The Brix degrees are measured using an infrared sensor. This measurement does not equate to Brix degrees from a density or refractive index measurement, because it will specifically measure dissolved sugar concentration instead of all dissolved solids. When using a refractometer, one should report the result as «refractometric dried substance» (RDS). One might speak of a liquid as having 20 °Bx RDS. This refers to a measure of percent by weight of total dried solids and, although not technically the same as Brix degrees determined through an infrared method, renders an accurate measurement of sucrose content, since sucrose in fact forms the majority of dried solids. The advent of in-line infrared Brix measurement sensors has made measuring the amount of dissolved sugar in products economical using a direct measurement.
Consumption[edit]
Refined sugar was a luxury before the 18th century. It became widely popular in the 18th century, then graduated to becoming a necessary food in the 19th century. This evolution of taste and demand for sugar as an essential food ingredient unleashed major economic and social changes.[23] Eventually, table sugar became sufficiently cheap and common enough to influence standard cuisine and flavored drinks.
Sucrose forms a major element in confectionery and desserts. Cooks use it for sweetening. It can also act as a food preservative when used in sufficient concentrations. Sucrose is important to the structure of many foods, including biscuits and cookies, cakes and pies, candy, and ice cream and sorbets. It is a common ingredient in many processed and so-called «junk foods».
Nutritional information[edit]
Sugars, granulated [sucrose]
| Nutritional value per 100 g (3.5 oz) | |
|---|---|
| Energy | 1,620 kJ (390 kcal) |
|
Carbohydrates |
100 g |
|
Fat |
0 g |
|
Protein |
0 g |
| Vitamins | Quantity
%DV† |
| Thiamine (B1) |
0% 0 mg |
| Riboflavin (B2) |
0% 0 mg |
| Niacin (B3) |
0% 0 mg |
| Vitamin C |
0% 0 mg |
| Minerals | Quantity
%DV† |
| Iron |
0% 0 mg |
| Phosphorus |
0% 0 mg |
| Potassium |
0% 2.0 mg |
| Selenium |
1% 0.6 μg |
|
Link to USDA Database entry |
|
|
|
| †Percentages are roughly approximated using US recommendations for adults. Source: USDA FoodData Central |
Fully refined sugar is 99.9% sucrose, thus providing only carbohydrate as dietary nutrient and 390 kilocalories per 100 g serving (USDA data, right table).[40] There are no micronutrients of significance in fully refined sugar (right table).[40]
Metabolism of sucrose[edit]
In humans and other mammals, sucrose is broken down into its constituent monosaccharides, glucose and fructose, by sucrase or isomaltase glycoside hydrolases, which are located in the membrane of the microvilli lining the duodenum.[41][42] The resulting glucose and fructose molecules are then rapidly absorbed into the bloodstream. In bacteria and some animals, sucrose is digested by the enzyme invertase. Sucrose is an easily assimilated macronutrient that provides a quick source of energy, provoking a rapid rise in blood glucose upon ingestion. Sucrose, as a pure carbohydrate, has an energy content of 3.94 kilocalories per gram (or 17 kilojoules per gram).
If consumed excessively, sucrose may contribute to the development of metabolic syndrome, including increased risk for type 2 diabetes, weight gain and obesity in adults and children.[43][44]
Tooth decay[edit]
Tooth decay (dental caries) has become a pronounced health hazard associated with the consumption of sugars, especially sucrose. Oral bacteria such as Streptococcus mutans live in dental plaque and metabolize any free sugars (not just sucrose, but also glucose, lactose, fructose, and cooked starches)[45] into lactic acid. The resultant lactic acid lowers the pH of the tooth’s surface, stripping it of minerals in the process known as tooth decay.[46][47]
All 6-carbon sugars and disaccharides based on 6-carbon sugars can be converted by dental plaque bacteria into acid that demineralizes teeth, but sucrose may be uniquely useful to Streptococcus sanguinis (formerly Streptococcus sanguis) and Streptococcus mutans.[48][49] Sucrose is the only dietary sugar that can be converted to sticky glucans (dextran-like polysaccharides) by extracellular enzymes. These glucans allow the bacteria to adhere to the tooth surface and to build up thick layers of plaque. The anaerobic conditions deep in the plaque encourage the formation of acids, which leads to carious lesions. Thus, sucrose could enable S. mutans, S. sanguinis and many other species of bacteria to adhere strongly and resist natural removal, e.g. by flow of saliva, although they are easily removed by brushing. The glucans and levans (fructose polysaccharides) produced by the plaque bacteria also act as a reserve food supply for the bacteria.
Such a special role of sucrose in the formation of tooth decay is much more significant in light of the almost universal use of sucrose as the most desirable sweetening agent. Widespread replacement of sucrose by high-fructose corn syrup (HFCS) has not diminished the danger from sucrose. If smaller amounts of sucrose are present in the diet, they will still be sufficient for the development of thick, anaerobic plaque and plaque bacteria will metabolise other sugars in the diet,[49] such as the glucose and fructose in HFCS.
Glycemic index[edit]
Sucrose is a disaccharide made up of 50% glucose and 50% fructose and has a glycemic index of 65.[50] Sucrose is digested rapidly,[51][52] but has a relatively low glycemic index due to its content of fructose, which has a minimal effect on blood glucose.[51]
As with other sugars, sucrose is digested into its components via the enzyme sucrase to glucose (blood sugar). The glucose component is transported into the blood where it serves immediate metabolic demands, or is converted and reserved in the liver as glycogen.[52]
Gout[edit]
The occurrence of gout is connected with an excess production of uric acid. A diet rich in sucrose may lead to gout as it raises the level of insulin, which prevents excretion of uric acid from the body. As the concentration of uric acid in the body increases, so does the concentration of uric acid in the joint liquid and beyond a critical concentration, the uric acid begins to precipitate into crystals. Researchers have implicated sugary drinks high in fructose in a surge in cases of gout.[53][54]
Sucrose intolerance[edit]
UN dietary recommendation[edit]
In 2015, the World Health Organization published a new guideline on sugars intake for adults and children, as a result of an extensive review of the available scientific evidence by a multidisciplinary group of experts. The guideline recommends that both adults and children ensure their intake of free sugars (monosaccharides and disaccharides added to foods and beverages by the manufacturer, cook or consumer, and sugars naturally present in honey, syrups, fruit juices and fruit juice concentrates) is less than 10% of total energy intake. A level below 5% of total energy intake brings additional health benefits, especially with regards to dental caries.[55]
Religious concerns[edit]
The sugar refining industry often uses bone char (calcinated animal bones) for decolorizing.[56][57] About 25% of sugar produced in the U.S. is processed using bone char as a filter, the remainder being processed with activated carbon. As bone char does not seem to remain in finished sugar, Jewish religious leaders consider sugar filtered through it to be pareve, meaning that it is neither meat nor dairy and may be used with either type of food. However, the bone char must source to a kosher animal (e.g. cow, sheep) for the sugar to be kosher.[57]
Trade and economics[edit]
One of the most widely traded commodities in the world throughout history, sugar accounts for around 2% of the global dry cargo market.[citation needed] International sugar prices show great volatility, ranging from around 3 cents to over 60 cents[clarification needed] per pound in the past 50 years. About 100 of the world’s 180 countries produce sugar from beet or cane, a few more refine raw sugar to produce white sugar, and all countries consume sugar. Consumption of sugar ranges from around 3 kilograms (7 lb) per person per annum in Ethiopia to around 40 kg (90 lb) in Belgium.[citation needed] Consumption per capita rises with income per capita until it reaches a plateau of around 35 kg (75 lb) per person per year in middle income countries.
Many countries subsidize sugar production heavily. The European Union, the United States, Japan, and many developing countries subsidize domestic production and maintain high tariffs on imports. Sugar prices in these countries have often up to triple the prices on the international market; today, with world market sugar futures prices currently strong, such prices were typically double world prices.
World raw sugar price 1960–2014
Within international trade bodies, especially in the World Trade Organization (WTO), the «G20» countries led by Brazil have long argued that, because these sugar markets in essence exclude cane sugar imports, the G20 sugar producers receive lower prices than they would under free trade. While both the European Union and United States maintain trade agreements whereby certain developing and least developed countries (LDCs) can sell certain quantities of sugar into their markets, free of the usual import tariffs, countries outside these preferred trade régimes have complained that these arrangements violate the «most favoured nation» principle of international trade. This has led to numerous tariffs and levies in the past.
In 2004, the WTO sided with a group of cane sugar exporting nations (led by Brazil and Australia) and ruled illegal the EU sugar-régime and the accompanying ACP-EU Sugar Protocol, that granted a group of African, Caribbean, and Pacific countries receive preferential access to the European sugar market.[58] In response to this and to other rulings of the WTO, and owing to internal pressures against the EU sugar-régime, the European Commission proposed on 22 June 2005 a radical reform of the EU sugar-régime that cut prices by 39% and eliminated all EU sugar exports.[59]
The African, Caribbean, Pacific and LDC sugar exporters reacted with dismay to the EU sugar proposals.[60] On 25 November 2005, the EC agreed to cut EU sugar prices by 36% as from 2009.
In 2007, it seemed[61] that the U.S. Sugar Program could become the next target for reform. However, some commentators expected heavy lobbying from the U.S. sugar industry, which donated $2.7 million to U.S. House and Senate incumbents in the 2006 U.S. election, more than any other group of U.S. food-growers.[62] Especially prominent among sugar lobbyists were the Fanjul Brothers, so-called «sugar barons» who made the single largest individual contributions of soft money to both the Democratic and Republican parties in the U.S. political system.[63][64]
Small quantities of sugar, especially specialty grades of sugar, reach the market as ‘fair trade’ commodities; the fair trade system produces and sells these products with the understanding that a larger-than-usual fraction of the revenue will support small farmers in the developing world. However, whilst the Fairtrade Foundation offers a premium of $60.00 per tonne to small farmers for sugar branded as «Fairtrade»,[65] government schemes such as the U.S. Sugar Program and the ACP-EU Sugar Protocol offer premiums of around $400.00 per tonne above world market prices. However, the EU announced on 14 September 2007 that it had offered «to eliminate all duties and quotas on the import of sugar into the EU».[66]
The U.S. Sugar Association subsequently launched a campaign to promote sugar over artificial substitutes. The Association now aggressively contradicts many common beliefs regarding negative side-effects of sugar consumption. The campaign aired a high-profile television commercial during the 2007 Primetime Emmy Awards on FOX Television.
References[edit]
- ^ Sucrose, International Chemical Safety Card 1507, Geneva: International Programme on Chemical Safety, November 2003
- ^ CRC Handbook of Chemistry and Physics, 49th edition, 1968–1969, p. D-188.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. «#0574». National Institute for Occupational Safety and Health (NIOSH).
- ^ «57-50-1 — CZMRCDWAGMRECN-UGDNZRGBSA-N — Sucrose [JAN:NF]». ChemIDplus. Archived from the original on 2014-08-12. Retrieved 2014-08-10.
- ^ a b Marcy Nicholson (17 November 2017). «World 2017/18 sugar production, consumption seen at record: USDA». Reuters. Archived from the original on 14 September 2019. Retrieved 21 December 2019.
- ^ Ryan KJ, Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 978-0-8385-8529-0.
- ^ William Allen Miller, Elements of Chemistry: Theoretical and Practical, Part III. Organic Chemistry (London, England: John W. Parker and son, 1857), pages 52 and 54 Archived 2017-04-24 at the Wayback Machine.
- ^ Marcellin Berthelot, Chimie organique fondée sur la synthèse (Paris, France: Mallet-Bachelier, 1860), pp. 254–55 Archived 2014-06-27 at the Wayback Machine.
- ^ Beevers, C. A.; McDonald, T. R. R.; Robertson, J. H.; Stern, F. (1952). «The crystal structure of sucrose». Acta Crystallogr. 5 (5): 689–90. doi:10.1107/S0365110X52001908.
- ^ Hynes, R. C.; Le Page, Y. (1991). «Sucrose, a convenient test crystal for absolute structures». Journal of Applied Crystallography. 24 (4): 352. doi:10.1107/S0021889891002492.
- ^ Nakka, Richard. «Potassium Nitrate/Sucrose Propellant (KNSU)». Experimental Rocketry. Archived from the original on 2015-10-26. Retrieved 2015-11-19.
- ^ Nakka, Richard. «KN-Sucrose (KNSU) Propellant Chemistry and Performance Characteristics». Experimental Rocketry. Archived from the original on 2014-11-18. Retrieved 2014-08-21.
- ^ «Sucrase» Archived 2010-04-25 at the Wayback Machine, Encyclopædia Britannica Online
- ^ All three from CRC Handbook of Chemistry and Physics, 49th edition, 1968-1969, pp. D-184-189.
- ^ Lemieux, R. U.; Huber, G. (1953). «A chemical synthesis of sucrose». J. Am. Chem. Soc. 75 (16): 4118. doi:10.1021/ja01112a545.
- ^ John E. Lunn (December 2008). «Sucrose Metabolism». Encyclopedia of Life Sciences. eLS. John Wiley & Sons Ltd. doi:10.1002/9780470015902.a0021259. ISBN 978-0470016176.
- ^ «Foods highest in Sucrose». SelfNutritiondata. Condé Nast. Archived from the original on 2015-07-19.
- ^ Douglas M. Considine (1982). Considine, Douglas M; Considine, Glenn D (eds.). Foods and Food Production Encyclopedia (1 ed.). Van Nostrand Reinhold Company Inc. p. 956. doi:10.1007/978-1-4684-8511-0. ISBN 978-1-4684-8513-4.
- ^ a b «Forced Labour». The National Archives, Government of the United Kingdom. 2010. Archived from the original on 2016-12-04.
- ^
Adas, Michael (2001). Agricultural and Pastoral Societies in Ancient and Classical History Archived 2013-06-14 at the Wayback Machine. Temple University Press. ISBN 1-56639-832-0. p. 311. - ^ a b Rolph, George (1873). Something about sugar: its history, growth, manufacture and distribution. San Francisco, J. J. Newbegin.
- ^ «Sugarcane: Saccharum Offcinarum» (PDF). USAID, Govt of United States. 2006. p. 7.1. Archived from the original (PDF) on 2013-11-06.
- ^ a b Mintz, Sidney (1986). Sweetness and Power: The Place of Sugar in Modern History. Penguin. ISBN 978-0-14-009233-2.
- ^ Lai, Walton (1993). Indentured labor, Caribbean sugar: Chinese and Indian migrants to the British West Indies, 1838–1918. ISBN 978-0-8018-7746-9.
- ^ Vertovik, Steven (1995). Cohen, Robin (ed.). The Cambridge survey of world migration. pp. 57–68. ISBN 978-0-521-44405-7.
- ^ Laurence, K (1994). A Question of Labour: Indentured Immigration Into Trinidad & British Guiana, 1875–1917. St Martin’s Press. ISBN 978-0-312-12172-3.
- ^ «St. Lucia’s Indian Arrival Day». Caribbean Repeating Islands. 2009-05-07. Archived from the original on 2017-04-24.
- ^ «Indian indentured labourers». The National Archives, Government of the United Kingdom. 2010. Archived from the original on 2011-12-12.
- ^ Marggraf (1747) «Experiences chimiques faites dans le dessein de tirer un veritable sucre de diverses plantes, qui croissent dans nos contrées» Archived 2016-06-24 at the Wayback Machine [Chemical experiments made with the intention of extracting real sugar from diverse plants that grow in our lands], Histoire de l’académie royale des sciences et belles-lettres de Berlin, pp. 79–90.
- ^ «Agribusiness Handbook: Sugar beet white sugar» (PDF). Food and Agriculture Organization, United Nations. 2009. Archived (PDF) from the original on 2015-09-05.
- ^ a b «Sugar: World Markets and Trade» (PDF). Office of Global Analysis, Foreign Agricultural Service, US Department of Agriculture. 4 November 2018. Archived (PDF) from the original on 21 October 2021. Retrieved 21 December 2018.
- ^ a b c d e Webb, Densie (7 June 2017). «Is High-Fructose Corn Syrup Worse Than Regular Sugar?». Berkeley Wellness, University of California — Berkeley. Archived from the original on 6 August 2019. Retrieved 19 September 2019.
- ^ a b c Parker-Pope, Tara (20 September 2010). «In Worries About Sweeteners, Think of All Sugars». New York Times. Archived from the original on 12 June 2018. Retrieved 2 January 2018.
- ^ a b «High Fructose Corn Syrup: Questions and Answers». US Food and Drug Administration. 5 November 2014. Archived from the original on 25 January 2018. Retrieved 2 January 2018.
- ^ «Top Sugarcane Producing Countries: Brazil outperforms its next 6 closest competitors combined». World Atlas. 25 April 2017. Archived from the original on 3 January 2018. Retrieved 2 January 2018.
- ^ January 2010 Newsletter Archived 2010-09-24 at the Wayback Machine, IBS Treatment Center
- ^ Steindl, Roderick (2005). Hogarth, DM (ed.). Syrup Clarification for Plantation White Sugar to meet New Quality Standards (PDF). Proceedings of the XXV Congress of International Society of Sugar Cane Technologists. Guatemala, Guatemala City. pp. 106–16. Archived (PDF) from the original on 2013-08-10.
- ^ a b Sugar Crystal Challenge Archived 2013-05-08 at the Wayback Machine. IEEE
- ^ «castor, n.2.» OED Online. Oxford University Press, June 2017. Web. 25 July 2017. It says castor is a misspelling that is now the preferred spelling.
- ^ a b «Nutrition Facts for sugars, granulated [sucrose] per 100 g (USDA National Nutrient Database, SR-21)». Conde Nast. 2014. Archived from the original on 7 March 2015. Retrieved 6 March 2015.
- ^ Gray GM (1971). «Intestinal digestion and maldigestion of dietary carbohydrate». Annual Review of Medicine. 22: 391–404. doi:10.1146/annurev.me.22.020171.002135. PMID 4944426.
- ^ Kaneko J.J. (2008) «Carbohydrate metabolism and its diseases» Archived 2014-09-22 at the Wayback Machine, p. 46 in Kaneko J.J., Harvey J.W., Bruss M.L. (eds.) Clinical Biochemistry of Domestic Animals, San Diego, CA: Academic Press, ISBN 012370491X.
- ^ Malik, V. S.; Popkin, B. M.; Bray, G. A.; Despres, J.-P.; Willett, W. C.; Hu, F. B. (2010). «Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis». Diabetes Care. 33 (11): 2477–83. doi:10.2337/dc10-1079. PMC 2963518. PMID 20693348.
- ^ Malik, Vasanti S.; Pan, An; Willett, Walter C.; Hu, Frank B. (2013-10-01). «Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis». The American Journal of Clinical Nutrition. 98 (4): 1084–1102. doi:10.3945/ajcn.113.058362. ISSN 0002-9165. PMC 3778861. PMID 23966427. Archived from the original on 2018-01-09. Retrieved 2018-12-21.
- ^ «What causes tooth decay?». Animated-teeth.com. Archived from the original on 2010-02-09. Retrieved 2010-05-05.
- ^ Tooth Decay Archived 2014-10-25 at the Wayback Machine. Elmhurst.edu. Retrieved on 2011-11-18.
- ^ What causes tooth decay? Archived 2010-02-09 at the Wayback Machine. Animated-teeth.com. Retrieved on 2011-11-18.
- ^ Tanzer, JM (August 1979). «Essential dependence of smooth surface caries on, and augmentation of fissure caries by, sucrose and Streptococcus mutans infection». Infection and Immunity. 25 (2): 526–31. doi:10.1128/IAI.25.2.526-531.1979. PMC 443577. PMID 489122.
- ^ a b Darlington, W. (August 1979). Metabolism of sucrose by Stepococcus sanguis 804 (NCTC 10904) and its relevance to the oral environment (Ph.D Thesis). University of Glasgow.
- ^ Wolever, Thomas M. S. (2006). The Glycaemic Index: A Physiological Classification of Dietary Carbohydrate. CABI. p. 64. ISBN 9781845930523. Archived from the original on 2017-12-16.
- ^ a b Wolever, Thomas M. S. (2006). The Glycaemic Index: A Physiological Classification of Dietary Carbohydrate. CABI. p. 65. ISBN 9781845930523. Archived from the original on 2017-12-16.
- ^ a b Food and Nutrition Board, Institute of Medicine of the US National Academies (2005). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). National Academies Press. p. 323. doi:10.17226/10490. ISBN 978-0-309-08525-0. Archived from the original on 2015-07-15.
- ^ Gout surge blamed on sweet drinks Archived 2009-08-16 at the Wayback Machine, BBC News, 1 February 2008
- ^ Magidenko, Leonid (2007-07-30). «Nutrients for Gout – good and bad». ABCVitaminsLife.com. Archived from the original on 2009-02-23. Retrieved 2010-05-05.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ^ See Guideline: Sugars intake for adults and children. Geneva: World Health Organization; 2015 Archived 2015-08-17 at the Wayback Machine
- ^ The Great Sugar Debate: Is it Vegan? Archived 2009-09-19 at the Wayback Machine. Vegfamily.com. Retrieved on 2011-11-18.
- ^ a b Yacoubou, MS, Jeanne (2007). «Is Your Sugar Vegan? An Update on Sugar Processing Practices» (PDF). Vegetarian Journal. Baltimore, MD: The Vegetarian Resource Group. 26 (4): 16–20. Archived (PDF) from the original on 2008-04-09. Retrieved 2007-04-04.
- ^ EC export subsidies on sugar (PDF). wto.org (Report). World Trade Organization. Archived from the original (PDF) on 2009-04-10. Retrieved 2011-11-18.
- ^ «Sugar». ec.europa.eu. Agriculture. European Commission. 2004-07-14. Archived from the original on 2009-08-22. Retrieved 2011-11-18.
- ^ The Fiji Communiqué on Sugar. acpsec.org (Report). ACP Group of States. 2007-05-03. Archived from the original on 2011-07-24. Retrieved 2011-11-18.
- ^ «International Sugar Trade Coalition». sugarcoalition.org (main page). Archived from the original on 2009-06-01. Retrieved 2011-11-18.
- ^ «Seeing sugar’s future in fuel». Business. The New York Times. 8 October 2007. Archived from the original on 2017-07-07.
- ^ «America’s sugar daddies». The New York Times. 11 November 2003. Archived from the original on 2016-03-07.
- ^ «Sugar daddie$». Mother Jones. 1997-05-01. Archived from the original on 2008-12-02. Retrieved 2010-05-05.
- ^ «Sugar». fairtrade.net. Fairtrade Labelling Organizations International (FLO). Archived from the original on 2012-08-02.
- ^ Trade Issues. External Trade. Ec.europa.eu (Report). European Commission. 2010-05-06. Archived from the original on 2009-09-05. Retrieved 2011-11-18.
Further reading[edit]
- Yudkin, J.; Edelman, J.; Hough, L. (1973). Sugar: Chemical, Biological and Nutritional Aspects of Sucrose. Butterworth. ISBN 978-0-408-70172-3.
External links[edit]
Wikimedia Commons has media related to Sucrose.
- 3D images of sucrose
- CDC – NIOSH Pocket Guide to Chemical Hazards
Сахароза — органическое вещество с кристаллической решеткой. Другое название — сахар. Это дисахарид, образованный остатками двух моносахаридов — фруктозы и глюкозы.
Узнаем больше о сахарозе, ее строении, формуле, физических и химических свойствах и о том, какую пользу играет она для живых организмов.
Формула и строение сахарозы
Структурная формула — C12H22O11, хотя она происходит от соединения двух простых сахаров, таких как глюкоза и фруктоза.
Два кольца этих сахаров объединены отдельным атомом кислорода, соединенным с двумя атомами углерода в цепочке. Другое расширение атома в молекуле также присутствует, главным образом, в комбинациях кислорода и водорода.
Связь между моносахаридами имеет O-глюкозидный тип. Кроме того, эта связь является дикарбонильной.
Физические свойства
По своим физическим свойствам она обладает сладким вкусом, может кристаллизоваться и растворима в воде.
Когда сахароза достигает желудка, она подвергается кислотному гидролизу и разлагается на части: на глюкозу и фруктозу. Остальная часть сахарозы переходит в тонкий кишечник, где ферментная сахароза преобразует ее в глюкозу и фруктозу.
Подчеркиваются ее специфические свойства в качестве питательного вещества для организма человека: она легко усваивается и не выделяет токсичных веществ. Это означает, что сахароза обладает как свойствами глюкозы, так и свойствами фруктозы, что означает, что она является источником энергии для организма.
Существует множество противоречий по поводу вреда, наносимого потреблением сахарозы, и несколько теорий по этому поводу. Основные дебаты сосредоточены на развитии кариеса, диабета, ожирения, атеросклероза и других патологий.
Интересно, что сахароза является триболюминесцентной, производящей свет механическим действием.
Благодаря низкой температуре плавления 1860С она очень быстро становится жидкой, очень легко прилипает к контейнеру, в котором она находится, может запросто обжечь кожу, если не соблюдать меры безопасности. Температура кипения раствора равна 101,40С.
Основные свойства описаны в таблице:
|
Физические свойства сахарозы |
|
|
Молярная масса |
342,3 г/моль |
|
Температура плавления |
1860С |
|
Растворимость |
211,5 г / 100 мл |
|
Плотность |
1,587 г/см3 |
Химические свойства
В молекуле сахарозы есть гидроксильные группы. Рассмотрим основные уравнения химических реакций сахарозы.
Реакция гидролиза
Сахароза способна подвергаться гидролизу, во время которого она распадается на моносахариды (глюкозу и фруктозу):
C12H22O11
+ H2O → C6H12O6
+ C6H12O6.
Реакции окисления
Дисахариды, которые сохраняют полуацетальный гидроксил, называются восстанавливающими. Дисахариды без полуацетального гидроксила называются невосстанавлиающими. Сахароза не является альдегидом.
Пример каталитического окисления сахарозы кислородом воздуха:
C12H22O11
+ 12 O2 → 12 CO2 + 11 H2O.
Качественная реакция с гидроксидом меди
Как провести лабораторный опыт:
-
лабораторное оборудование: пробирка, горелка;
-
реагенты: водный раствор сахарозы, гидроксид меди (II);
-
действия: залейте раствор сахарозы в пробирку, добавьте гидроксид меди (II), разогрейте пробирку над горелкой:
C12H22O11
+ 2 Cu (OH)2 → ярко-синее окрашивание;
Замечания: синий осадок не изменил цвет, несмотря на подачу энергии в виде тепла;
Вывод: сахароза не обладает восстановительными свойствами.
Нахождение сахарозы в природе
Она обычно извлекается из сахарного тростника, свеклы или кукурузы.
Сахарный тростник
Другими коммерческими (незначительными) источниками являются сладкий сорго и кленовый сироп.
Получение сахарозы
Сахароза извлекается из сырья, в котором она содержится, а затем очищается и кристаллизуется.
Применение и биологическая роль сахарозы
Широкое применение сахарозы обусловлено ее способностью к подслащению и свойствами функциональной консистенции. По этой причине она важна для структуры некоторых продуктов питания, особенно кондитерских изделий.
Также является вспомогательным компонентом в сохранении пищи, будучи добавкой, широко используемой в приготовлении так называемой нездоровой пищи.
В проросших семенах растений жиры и белки, находящиеся на хранении, превращаются в сахарозу для транспортировки в процессе развития растений.
Главная функция сахарозы в организме человека — она помогает вырабатывать энергию, необходимую для функционирования различных органов.
Примером наиболее распространенных в природе дисахаридов (олигосахаридом) является сахароза (свекловичный или тростниковый сахар).
Биологическая роль сахарозы
Наибольшее значение в питании человека имеет сахароза, которая в значительном количестве поступает в организм с пищей. Подобно глюкозе и фруктозе сахароза после расщепления ее в кишечнике быстро всасывается из желудочно-кишечного тракта в кровь и легко используется как источник энергии.
Важнейший пищевой источник сахарозы — сахар.
Строение сахарозы
Молекулярная формула сахарозы С12Н22О11.
Сахароза имеет более сложное строение, чем глюкоза. Молекула сахарозы состоит из остатков молекул глюкозы и фруктозы в их циклической форме. Они соединены друг с другом за счет взаимодействия полуацетальных гидроксилов (1→2) -гликозидной связью, то есть свободный полуацетальный (гликозидный) гидроксил отсутствует:
Сахароза. Строение
Физические свойства сахарозы и нахождение в природе
Сахароза (обыкновенный сахар) – белое кристаллическое вещество, более сладкое, чем глюкоза, хорошо растворимое в воде.
Температура плавления сахарозы 160°C. При застывании расплавленной сахарозы образуется аморфная прозрачная масса – карамель.
Сахароза является весьма распространённым в природе дисахаридом, она встречается во многих фруктах, плодах и ягодах. Особенно много ее содержится в сахарной свёкле (16-21%) и сахарном тростнике (до 20%), которые и используются для промышленного производства пищевого сахара.
Содержание сахарозы в сахаре 99,5%. Сахар часто называют «носителем пустых калорий», так как сахар – это чистый углевод и не содержит других питательных веществ, таких, как, например, витамины, минеральные соли.
Химические свойства
Для сахарозы характерны реакции по гидроксильным группам.
1. Качественная реакция с гидроксидом меди (II)
Наличие гидроксильных групп в молекуле сахарозы легко подтверждается реакцией с гидроксидами металлов.
Видеоопыт «Доказательство наличия гидроксильных групп в сахарозе»
Если раствор сахарозы прилить к гидроксиду меди (II), образуется ярко-синий раствор сахарата меди (качественная реакция многоатомных спиртов):
2. Реакция окисления
Восстанавливающие дисахариды
Дисахариды, в молекулах которых сохраняется полуацетальный (гликозидный) гидроксил (мальтоза, лактозы), в растворах частично превращаются из циклических форм в открытые альдегидные формы и вступают в реакции, характерные для альдегидов: реагируют с аммиачным раствором оксида серебра и восстанавливают гидроксид меди (II) до оксида меди (I). Такие дисахариды называются восстанавливающими (восстанавливают Cu (OH)2 и Ag2O).
Реакция «серебряного зеркала»
Реакция с гидроксидом меди (II)
Невосстанавливающий дисахарид
Дисахариды, в молекулах которых нет полуацетального (гликозидного) гидроксила (сахароза) и которые не могут переходить в открытые карбонильные формы, называются невосстанавливающими (не восстанавливают Cu (OH)2 и Ag2O).
Сахароза, в отличие от глюкозы, не является альдегидом. Сахароза, находясь в растворе, не вступает в реакцию «серебряного зеркала» и при нагревании с гидроксидом меди (II) не образует красного оксида меди (I), так как не способна превращаться в открытую форму, содержащую альдегидную группу.
Видеоопыт «Отсутствие восстанавливающей способности сахарозы»
3. Реакция гидролиза
Для дисахаридов характерна реакция гидролиза (в кислой среде или под действием ферментов), в результате которой образуются моносахариды.
Сахароза способна подвергаться гидролизу (при нагревании в присутствии ионов водорода). При этом из одной молекулы сахарозы образуется молекула глюкозы и молекула фруктозы:
Видеоопыт «Кислотный гидролиз сахарозы»
Мальтоза и лактоза при гидролизе расщепляются на составляющие их моносахариды за счёт разрыва связей между ними (гликозидных связей):
Таким образом, реакция гидролиза дисахаридов является обратной процессу их образования из моносахаридов.
В живых организмах гидролиз дисахаридов происходит при участии ферментов.
Получение сахарозы
Сахарную свеклу или сахарный тростник превращают в тонкую стружку и помещают в диффузоры (огромные котлы), в которых горячая вода вымывает сахарозу (сахар).
Вместе с сахарозой в водный раствор переходят и другие компоненты (различные органические кислоты, белки, красящие вещества и др.). чтобы отделить эти продукты от сахарозы, раствор обрабатывают известковым молоком (гидроксидом кальция). В результате этого образуются малорастворимые соли, которые выпадают в осадок. Сахароза образует с гидроксидом кальция растворимый сахарат кальция С12Н22О11·CaO·2Н2О.
Для разложения сахарата кальция и нейтрализации избытка гидроксида кальция через раствор пропускают оксид углерода ( IV).
Выпавший в осадок карбонат кальция отфильтровывают, а раствор упаривают в вакуумных аппаратах. По мере образования кристалликов сахара отделяют с помощью центрифуги. Оставшийся раствор – меласса – содержит до 50% сахарозы. Его используют для производства лимонной кислоты.
Выделенную сахарозу очищают и обесцвечивают. Для этого ее растворяют в воде и полученный раствор фильтруют через активированный уголь. Затем раствор снова упаривают и кристаллизуют.
Применение сахарозы
Сахароза в основном используется как самостоятельный продукт питания (сахар), а также при изготовлении кондитерских изделий, алкогольных напитков, соусов. Ее используют в высоких концентрациях в качестве консерванта. Путем гидролиза из нее получают искусственный мёд.
Сахароза находит применение в химической промышленности. С помощью ферментации из нее получают этанол, бутанол, глицерин, левулиновую и лимонную кислоты, декстран.
В медицине сахарозу используют при изготовлении порошков, микстур, сиропов, в том числе для новорожденных детей (для придания сладкого вкуса или консервации).
Углеводы
Олигосахариды. Дисахариды