базедова болезнь
- базедова болезнь
-
баз’едова бол’езнь, баз’едовой бол’езни
Русский орфографический словарь. / Российская академия наук. Ин-т рус. яз. им. В. В. Виноградова. — М.: «Азбуковник».
.
1999.
Смотреть что такое «базедова болезнь» в других словарях:
-
БАЗЕДОВА БОЛЕЗНЬ — преимущественно у женщин, выражающаяся сердцебиением, выпучиванием глаз и сильным развитием зоба. Названа по имени д ра Базедова, описавшего ее впервые в 1840 г. Полный словарь иностранных слов, вошедших в употребление в русском языке. Попов М.,… … Словарь иностранных слов русского языка
-
БАЗЕДОВА БОЛЕЗНЬ — (morbus Graves Basedowi). Описываемое заболевание было известно еще Морганьи (Morgagni, 1761 г.), Флайани (Flajani, 1802 г.), Парри (Parry, 1825 г.) и, гл. обр., Гревс (Graves) удовлетворительно изучили его симптоматологию. Мерзебургский врач… … Большая медицинская энциклопедия
-
БАЗЕДОВА БОЛЕЗНЬ — БАЗЕДОВА БОЛЕЗНЬ, то же, что зоб диффузный токсический … Современная энциклопедия
-
БАЗЕДОВА БОЛЕЗНЬ — то же, что зоб диффузный токсический … Большой Энциклопедический словарь
-
базедова болезнь — то же, что зоб диффузный токсический. * * * БАЗЕДОВА БОЛЕЗНЬ БАЗЕДОВА БОЛЕЗНЬ, то же, что зоб диффузный токсический (см. ЗОБ ДИФФУЗНЫЙ ТОКСИЧЕСКИЙ) … Энциклопедический словарь
-
базедова болезнь — Болезнь, вызываемая нарушением функции щитовидной железы и сопровождающаяся нередко пучеглазием; тиреотоксико/з. По фамилии врача К. Базедова (1799 1854), впервые описавшего эту болезнь … Словарь многих выражений
-
базедова болезнь — (диффузный токсический зоб, болезнь Грейвса, болезнь Флаяни, болезнь Перри) заболевание при котором имеется равномерное, диффузное увеличение щитовидной железы, избыточная продукция тиреиодных гормонов (гормонов щитовидной железы) и изменения в… … Медицинские термины
-
Базедова болезнь — этим именем называется заболевание, описанное впервые в 1840 г. Базедовым, имеющая следующие симптомы: сердцебиение, ускорение сердечной деятельности, усиленную пульсацию головных и шейных сосудов. Впоследствии к этим первоначальным симптомам… … Энциклопедический словарь Ф.А. Брокгауза и И.А. Ефрона
-
базедова болезнь — (morbus Basedowi; К. A. von Basedow, 1799 1854, нем. врач) см. Зоб диффузный токсический … Большой медицинский словарь
-
Базедова болезнь — заболевание, связанное с повышением функции щитовидной железы, описанное немецким врачом К. Базедовом (К. Basedow, 1799 1854), то же, что Зоб диффузный токсический … Большая советская энциклопедия
-
Базедова болезнь — I Базедова болезнь (morbus Basedowi; К. А. von Basedow, нем. врач, 1799 1854) см. Зоб диффузный токсический. II Базедова болезнь (morbus Basedowi; K.A. von Basedow, 1799 1854, нем. врач) см. Зоб диффузный токсический … Медицинская энциклопедия
| Graves’ disease | |
|---|---|
| Other names | Toxic diffuse goiter, Flajani–Basedow–Graves disease |
 |
|
| The classic finding of exophthalmos and lid retraction in Graves’ disease | |
| Specialty | Endocrinology |
| Symptoms | Enlarged thyroid, irritability, muscle weakness, sleeping problems, fast heartbeat, weight loss, poor tolerance of heat,[1] anxiety, tremor of hands or fingers, warm and moist skin, increased perspiration, goiter, changes in menstrual cycle, easy bruising, erectile dysfunction, reduced libido, frequent bowel movements, bulging eyes (Graves’ ophthalmopathy), thick red skin on shins or the top of foot (pretibial myxedema)[2] |
| Complications | Graves’ ophthalmopathy[1] |
| Causes | Unknown[3] |
| Risk factors | Family history, other autoimmune diseases[1] |
| Diagnostic method | Blood tests, radioiodine uptake[1][4] |
| Treatment | Radioiodine therapy, medications, thyroid surgery[1] |
| Frequency | 0.5% (males), 3% (females)[5] |
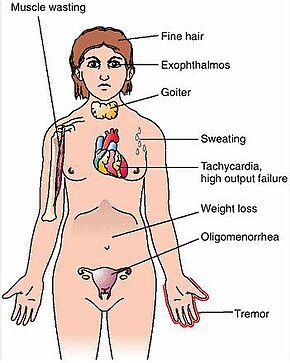
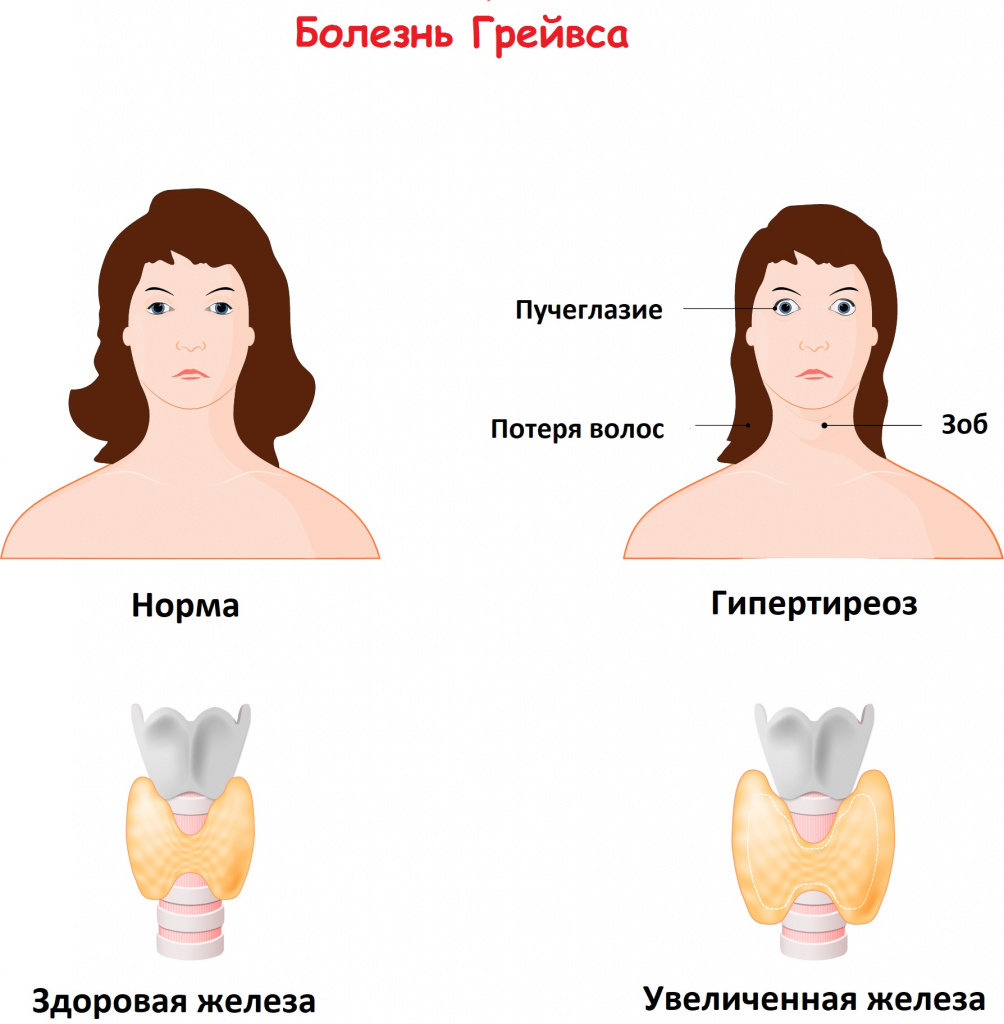
Graves’ disease (German: Morbus Basedow), also known as toxic diffuse goiter, is an autoimmune disease that affects the thyroid.[1] It frequently results in and is the most common cause of hyperthyroidism.[5] It also often results in an enlarged thyroid.[1] Signs and symptoms of hyperthyroidism may include irritability, muscle weakness, sleeping problems, a fast heartbeat, poor tolerance of heat, diarrhea and unintentional weight loss.[1] Other symptoms may include thickening of the skin on the shins, known as pretibial myxedema, and eye bulging, a condition caused by Graves’ ophthalmopathy.[1] About 25 to 30% of people with the condition develop eye problems.[1][4]
The exact cause of the disease is unclear; however, it is believed to involve a combination of genetic and environmental factors.[3] A person is more likely to be affected if they have a family member with the disease.[1] If one twin is affected, a 30% chance exists that the other twin will also have the disease.[6] The onset of disease may be triggered by physical or emotional stress, infection, or giving birth.[4] Those with other autoimmune diseases such as type 1 diabetes and rheumatoid arthritis are more likely to be affected.[1] Smoking increases the risk of disease and may worsen eye problems.[1] The disorder results from an antibody, called thyroid-stimulating immunoglobulin (TSI), that has a similar effect to thyroid stimulating hormone (TSH).[1] These TSI antibodies cause the thyroid gland to produce excess thyroid hormones.[1] The diagnosis may be suspected based on symptoms and confirmed with blood tests and radioiodine uptake.[1][4] Typically, blood tests show a raised T3 and T4, low TSH, increased radioiodine uptake in all areas of the thyroid and TSI antibodies.[4]
The three treatment options are radioiodine therapy, medications, and thyroid surgery.[1] Radioiodine therapy involves taking iodine-131 by mouth, which is then concentrated in the thyroid and destroys it over weeks to months.[1] The resulting hypothyroidism is treated with synthetic thyroid hormones.[1] Medications such as beta blockers may control some of the symptoms, and antithyroid medications such as methimazole may temporarily help people while other treatments are having effect.[1] Surgery to remove the thyroid is another option.[1] Eye problems may require additional treatments.[1]
Graves’ disease will develop in about 0.5% of males and 3% of females.[5] It occurs about 7.5 times more often in women than in men.[1] Often, it starts between the ages of 40 and 60 but can begin at any age.[6] It is the most common cause of hyperthyroidism in the United States (about 50 to 80% of cases).[1][4] The condition is named after Irish surgeon Robert Graves, who described it in 1835.[6] A number of prior descriptions also exist.[6]
Signs and symptoms[edit]
The signs and symptoms of Graves’ disease virtually all result from the direct and indirect effects of hyperthyroidism, with main exceptions being Graves’ ophthalmopathy, goiter, and pretibial myxedema (which are caused by the autoimmune processes of the disease). Symptoms of the resultant hyperthyroidism are mainly insomnia, hand tremor, hyperactivity, hair loss, excessive sweating, oligomenorrhea, itching, heat intolerance, weight loss despite increased appetite, diarrhea, frequent defecation, palpitations, periodic partial muscle weakness or paralysis in those especially of Asian descent,[7] and skin warmth and moistness.[8] Further signs that may be seen on physical examination are most commonly a diffusely enlarged (usually symmetric), nontender thyroid, lid lag, excessive lacrimation due to Graves’ ophthalmopathy, arrhythmias of the heart, such as sinus tachycardia, atrial fibrillation, and premature ventricular contractions, and hypertension.[8][9]
Cause[edit]
The exact cause is unclear; however, it is believed to involve a combination of genetic and environmental factors.[3] While a theoretical mechanism occurs by which exposure to severe stressors and high levels of subsequent distress such as PTSD (Post traumatic stress disorder) could increase the risk of immune disease and cause an aggravation of the autoimmune response that leads to Graves’ disease, more robust clinical data are needed for a firm conclusion.[10]
Genetics[edit]
A genetic predisposition for Graves’ disease is seen, with some people more prone to develop TSH receptor activating antibodies due to a genetic cause. Human leukocyte antigen DR (especially DR3) appears to play a role.[11] To date, no clear genetic defect has been found to point to a single-gene cause.[citation needed]
Genes believed to be involved include those for thyroglobulin, thyrotropin receptor, protein tyrosine phosphatase nonreceptor type 22 (PTPN22), and cytotoxic T-lymphocyte–associated antigen 4, among others.[12]
Infectious trigger[edit]
Since Graves’ disease is an autoimmune disease which appears suddenly, often later in life, a viral or bacterial infection may trigger antibodies which cross-react with the human TSH receptor, a phenomenon known as antigenic mimicry.[13]
The bacterium Yersinia enterocolitica bears structural similarity with the human thyrotropin receptor[11] and was hypothesized to contribute to the development of thyroid autoimmunity arising for other reasons in genetically susceptible individuals.[14]
In the 1990s, it was suggested that Y. enterocolitica may be associated with Graves’ disease.[15]
More recently, the role for Y. enterocolitica has been disputed.[16]
Epstein–Barr virus (EBV) is another potential trigger.[17]
Mechanism[edit]
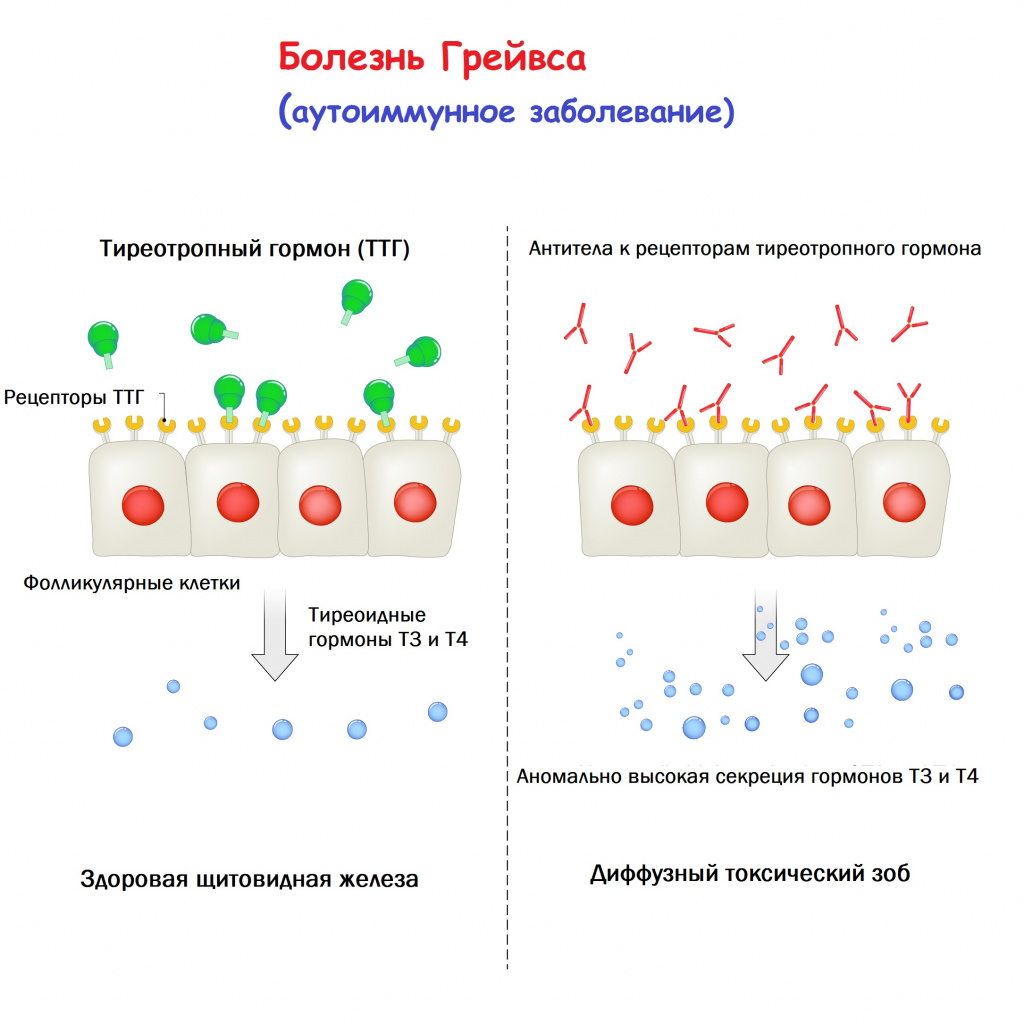
Thyroid-stimulating immunoglobulins recognize and bind to the thyrotropin receptor (TSH receptor) which stimulates the secretion of thyroxine (T4) and triiodothyronine (T3). Thyroxine receptors in the pituitary gland are activated by the surplus hormone, suppressing additional release of TSH in a negative feedback loop. The result is very high levels of circulating thyroid hormones and a low TSH level.[citation needed]
Pathophysiology[edit]
Histopathological image of diffuse hyperplasia of the thyroid gland (clinically presenting as hyperthyroidism)
Graves’ disease is an autoimmune disorder, in which the body produces antibodies that are specific to a self-protein: the receptor for thyroid-stimulating hormone. (Antibodies to thyroglobulin and to the thyroid hormones T3 and T4 may also be produced.)
These antibodies cause hyperthyroidism because they bind to the TSHr and chronically stimulate it. The TSHr is expressed on the thyroid follicular cells of the thyroid gland (the cells that produce thyroid hormone), and the result of chronic stimulation is an abnormally high production of T3 and T4. This, in turn, causes the clinical symptoms of hyperthyroidism, and the enlargement of the thyroid gland visible as goiter.
The infiltrative exophthalmos frequently encountered has been explained by postulating that the thyroid gland and the extraocular muscles share a common antigen which is recognized by the antibodies. Antibodies binding to the extraocular muscles would cause swelling behind the eyeball.
The «orange peel» skin has been explained by the infiltration of antibodies under the skin, causing an inflammatory reaction and subsequent fibrous plaques.
The three types of autoantibodies to the TSH receptor currently recognized are:
- Thyroid stimulating immunoglobulins: these antibodies (mainly IgG) act as long-acting thyroid stimulants (LATS), activating the cells through a slower and more drawn out process compared to TSH, leading to an elevated production of thyroid hormone.
- Thyroid growth immunoglobulins: these antibodies bind directly to the TSH receptor and have been implicated in the growth of thyroid follicles.
- Thyrotrophin binding-inhibiting immunoglobulins: these antibodies inhibit the normal union of TSH with its receptor.
- Some actually act as if TSH itself is binding to its receptor, thus inducing thyroid function.
- Other types may not stimulate the thyroid gland, but prevent TSI and TSH from binding to and stimulating the receptor.
Another effect of hyperthyroidism is bone loss from osteoporosis, caused by an increased excretion of calcium and phosphorus in the urine and stool. The effects can be minimized if the hyperthyroidism is treated early. Thyrotoxicosis can also augment calcium levels in the blood by as much as 25%. This can cause stomach upset, excessive urination, and impaired kidney function.[18]
Diagnosis[edit]
Graves’ disease may present clinically with one or more of these characteristic signs:[citation needed]
- Rapid heartbeat (80%)
- Diffuse palpable goiter with audible bruit (70%)
- Tremor (40%)
- Exophthalmos (protuberance of one or both eyes), periorbital edema (25%)
- Fatigue (70%), weight loss (60%) with increased appetite in young people and poor appetite in the elderly, and other symptoms of hyperthyroidism/thyrotoxicosis
- Heat intolerance (55%)
- Tremulousness (55%)
- Palpitations (50%)
Two signs are truly ‘diagnostic’ of Graves’ disease (i.e., not seen in other hyperthyroid conditions): exophthalmos and nonpitting edema (pretibial myxedema). Goiter is an enlarged thyroid gland and is of the diffuse type (i.e., spread throughout the gland). Diffuse goiter may be seen with other causes of hyperthyroidism, although Graves’ disease is the most common cause of diffuse goiter. A large goiter will be visible to the naked eye, but a small one (mild enlargement of the gland) may be detectable only by physical examination. Occasionally, goiter is not clinically detectable, but may be seen only with computed tomography or ultrasound examination of the thyroid.[citation needed]
Another sign of Graves’ disease is hyperthyroidism; that is, overproduction of the thyroid hormones T3 and T4. Normal thyroid levels are also seen, and occasionally also hypothyroidism, which may assist in causing goiter (though it is not the cause of the Graves’ disease). Hyperthyroidism in Graves’ disease is confirmed, as with any other cause of hyperthyroidism, by measuring elevated blood levels of free (unbound) T3 and T4.[citation needed]
Other useful laboratory measurements in Graves’ disease include thyroid-stimulating hormone (TSH, usually undetectable in Graves’ disease due to negative feedback from the elevated T3 and T4), and protein-bound iodine (elevated). Serologically detected thyroid-stimulating antibodies, radioactive iodine (RAI) uptake, or thyroid ultrasound with Doppler all can independently confirm a diagnosis of Graves’ disease.
Biopsy to obtain histiological testing is not normally required, but may be obtained if thyroidectomy is performed.
The goiter in Graves’ disease is often not nodular, but thyroid nodules are also common.[19] Differentiating common forms of hyperthyroidism such as Graves’ disease, single thyroid adenoma, and toxic multinodular goiter is important to determine proper treatment.[19] The differentiation among these entities has advanced, as imaging and biochemical tests have improved. Measuring TSH-receptor antibodies with the h-TBII assay has been proven efficient and was the most practical approach found in one study.[20]
Eye disease[edit]
Thyroid-associated ophthalmopathy (TAO), or thyroid eye disease (TED), is the most common extrathyroidal manifestation of Graves’ disease. It is a form of idiopathic lymphocytic orbital inflammation, and although its pathogenesis is not completely understood, autoimmune activation of orbital fibroblasts, which in TAO express the TSH receptor, is thought to play a central role.[21]
Hypertrophy of the extraocular muscles, adipogenesis, and deposition of nonsulfated glycosaminoglycans and hyaluronate, causes expansion of the orbital fat and muscle compartments, which within the confines of the bony orbit may lead to dysthyroid optic neuropathy, increased intraocular pressures, proptosis, venous congestion leading to chemosis and periorbital edema, and progressive remodeling of the orbital walls.[22][23][24] Other distinctive features of TAO include lid retraction, restrictive myopathy, superior limbic keratoconjunctivitis, and exposure keratopathy.[citation needed]
Severity of eye disease may be classified by the mnemonic: «NO SPECS»:[25]
- Class 0: No signs or symptoms
- Class 1: Only signs (limited to upper lid retraction and stare, with or without lid lag)
- Class 2: Soft tissue involvement (oedema of conjunctivae and lids, conjunctival injection, etc.)
- Class 3: Proptosis
- Class 4: Extraocular muscle involvement (usually with diplopia)
- Class 5: Corneal involvement (primarily due to lagophthalmos)
- Class 6: Sight loss (due to optic nerve involvement)
Typically the natural history of TAO follows Rundle’s curve, which describes a rapid worsening during an initial phase, up to a peak of maximum severity, and then improvement to a static plateau without, however, resolving back to a normal condition.[26]
Management[edit]
Treatment of Graves’ disease includes antithyroid drugs that reduce the production of thyroid hormone, radioiodine (radioactive iodine I-131) and thyroidectomy (surgical excision of the gland). As operating on a hyperthyroid patient is dangerous, prior to thyroidectomy, preoperative treatment with antithyroid drugs is given to render the patient euthyroid. Each of these treatments has advantages and disadvantages, and no single treatment approach is considered the best for everyone.[citation needed]
Treatment with antithyroid medications must be administered for six months to two years to be effective. Even then, upon cessation of the drugs, the hyperthyroid state may recur. The risk of recurrence is about 40–50%, and lifelong treatment with antithyroid drugs carries some side effects such as agranulocytosis and liver disease.[27] Side effects of the antithyroid medications include a potentially fatal reduction in the level of white blood cells. Therapy with radioiodine is the most common treatment in the United States, while antithyroid drugs and/or thyroidectomy are used more often in Europe, Japan, and most of the rest of the world.
β-Blockers (such as propranolol) may be used to inhibit the sympathetic nervous system symptoms of tachycardia and nausea until antithyroid treatments start to take effect. Pure β-blockers do not inhibit lid retraction in the eyes, which is mediated by alpha adrenergic receptors.
Antithyroid drugs[edit]
The main antithyroid drugs are carbimazole (in the UK), methimazole (in the US), and propylthiouracil/PTU. These drugs block the binding of iodine and coupling of iodotyrosines. The most dangerous side effect is agranulocytosis (1/250, more in PTU). Others include granulocytopenia (dose-dependent, which improves on cessation of the drug) and aplastic anemia. Patients on these medications should see a doctor if they develop sore throat or fever. The most common side effects are rash and peripheral neuritis. These drugs also cross the placenta and are secreted in breast milk. Lugol’s iodine may be used to block hormone synthesis before surgery.[citation needed]
A randomized control trial testing single-dose treatment for Graves’ found methimazole achieved euthyroid state more effectively after 12 weeks than did propylthyouracil (77.1% on methimazole 15 mg vs 19.4% in the propylthiouracil 150 mg groups).[28]
No difference in outcome was shown for adding thyroxine to antithyroid medication and continuing thyroxine versus placebo after antithyroid medication withdrawal. However, two markers were found that can help predict the risk of recurrence. These two markers are a positive TSHr antibody (TSHR-Ab) and smoking. A positive TSHR-Ab at the end of antithyroid drug treatment increases the risk of recurrence to 90% (sensitivity 39%, specificity 98%), and a negative TSHR-Ab at the end of antithyroid drug treatment is associated with a 78% chance of remaining in remission. Smoking was shown to have an impact independent to a positive TSHR-Ab.[29]
Radioiodine[edit]
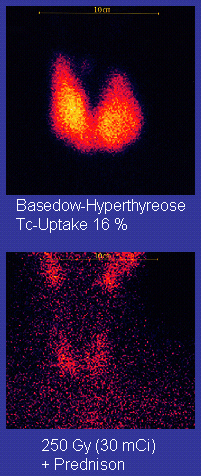
Scan of affected thyroid before (top) and after (bottom) radioiodine therapy
Radioiodine (radioactive iodine-131) was developed in the early 1940s at the Mallinckrodt General Clinical Research Center. This modality is suitable for most patients, although some prefer to use it mainly for older patients. Indications for radioiodine are failed medical therapy or surgery and where medical or surgical therapy are contraindicated. Hypothyroidism may be a complication of this therapy, but may be treated with thyroid hormones if it appears. The rationale for radioactive iodine is that it accumulates in the thyroid and irradiates the gland with its beta and gamma radiations, about 90% of the total radiation being emitted by the beta (electron) particles. The most common method of iodine-131 treatment is to administer a specified amount in microcuries per gram of thyroid gland based on palpation or radiodiagnostic imaging of the gland over 24 hours.[30] Patients who receive the therapy must be monitored regularly with thyroid blood tests to ensure they are treated with thyroid hormone before they become symptomatically hypothyroid.[31]
Contraindications to RAI are pregnancy (absolute), ophthalmopathy (relative; it can aggravate thyroid eye disease), or solitary nodules.[32]
Disadvantages of this treatment are a high incidence of hypothyroidism (up to 80%) requiring eventual thyroid hormone supplementation in the form of a daily pill(s). The radioiodine treatment acts slowly (over months to years) to destroy the thyroid gland, and Graves’ disease–associated hyperthyroidism is not cured in all persons by radioiodine, but has a relapse rate that depends on the dose of radioiodine which is administered.[32] In rare cases, radiation induced thyroiditis has been linked to this treatment.[33]
Surgery[edit]
This modality is suitable for young and pregnant people. Indications for thyroidectomy can be separated into absolute indications or relative indications. These indications aid in deciding which people would benefit most from surgery.[27] The absolute indications are a large goiter (especially when compressing the trachea), suspicious nodules or suspected cancer (to pathologically examine the thyroid), and people with ophthalmopathy and additionally if it is the person’s preferred method of treatment or if refusing to undergo radioactive iodine treatment. Pregnancy is advised to be delayed for 6 months after radioactive iodine treatment.[27]
Both bilateral subtotal thyroidectomy and the Hartley-Dunhill procedure (hemithyroidectomy on one side and partial lobectomy on other side) are possible.
Advantages are immediate cure and potential removal of carcinoma. Its risks are injury of the recurrent laryngeal nerve, hypoparathyroidism (due to removal of the parathyroid glands), hematoma (which can be life-threatening if it compresses the trachea), relapse following medical treatment, infections (less common), and scarring.[27] The increase in the risk of nerve injury can be due to the increased vascularity of the thyroid parenchyma and the development of links between the thyroid capsule and the surrounding tissues. Reportedly, a 1% incidence exists of permanent recurrent laryngeal nerve paralysis after complete thyroidectomy.[27] Risks related to anesthesia are many, thus coordination with the anesthesiologist and patient optimization for surgery preoperatively are essential. Removal of the gland enables complete biopsy to be performed to have definite evidence of cancer anywhere in the thyroid. (Needle biopsies are not so accurate at predicting a benign state of the thyroid). No further treatment of the thyroid is required, unless cancer is detected. Radioiodine uptake study may be done after surgery, to ensure all remaining (potentially cancerous) thyroid cells (i.e., near the nerves to the vocal cords) are destroyed. Besides this, the only remaining treatment will be levothyroxine, or thyroid replacement pills to be taken for the rest of the patient’s life.
A 2013 review article concludes that surgery appears to be the most successful in the management of Graves’ disease, with total thyroidectomy being the preferred surgical option.[34]
Eyes[edit]
Mild cases are treated with lubricant eye drops or nonsteroidal anti-inflammatory drops. Severe cases threatening vision (corneal exposure or optic nerve compression) are treated with steroids or orbital decompression. In all cases, cessation of smoking is essential. Double vision can be corrected with prism glasses and surgery (the latter only when the process has been stable for a while).
Difficulty closing eyes can be treated with lubricant gel at night, or with tape on the eyes to enable full, deep sleep.
Orbital decompression can be performed to enable bulging eyes to retreat back into the head. Bone is removed from the skull behind the eyes, and space is made for the muscles and fatty tissue to fall back into the skull. [35]
Eyelid surgery can be performed on upper and/or lower eyelids to reverse the effects of Graves’ disease[36] on the eyelids. Eyelid muscles can become tight with Graves’ disease, making it impossible to close the eyes all the way. Eyelid surgery involves an incision along the natural crease of the eyelid, and a scraping away of the muscle that holds the eyelid open. This makes the muscle weaker, which allows the eyelid to extend over the eyeball more effectively. Eyelid surgery helps reduce or eliminate dry eye symptoms.
For management of clinically active Graves’ disease, orbitopathy (clinical activity score >2) with at least mild to moderate severity, intravenous glucocorticoids are the treatment of choice, usually administered in the form of pulse intravenous methylprednisolone. Studies have consistently shown that pulse intravenous methylprednisolone is superior to oral glucocorticoids both in terms of efficacy and decreased side effects for managing Graves’ orbitopathy.[37]
Prognosis[edit]
If left untreated, more serious complications could result, including birth defects in pregnancy, increased risk of a miscarriage, bone mineral loss[38] and, in extreme cases, death. Graves’ disease is often accompanied by an increase in heart rate, which may lead to further heart complications, including loss of the normal heart rhythm (atrial fibrillation), which may lead to stroke. If the eyes are proptotic (bulging) enough that the lids do not close completely at night, dryness will occur – with the risk of a secondary corneal infection, which could lead to blindness. Pressure on the optic nerve behind the globe can lead to visual field defects and vision loss, as well. Prolonged untreated hyperthyroidism can lead to bone loss, which may resolve when treated.[38]
Epidemiology[edit]
Graves’ disease occurs in about 0.5% of people.[4] Graves’ disease data has shown that the lifetime risk for women is around 3% and 0.5% for men.[40] It occurs about 7.5 times more often in women than in men[1] and often starts between the ages of 40 and 60.[6] It is the most common cause of hyperthyroidism in the United States (about 50 to 80% of cases).[1][4]
History[edit]
Graves’ disease owes its name to the Irish doctor Robert James Graves,[41] who described a case of goiter with exophthalmos in 1835.[42] (Medical eponyms are often styled nonpossessively; thus Graves’ disease and Graves disease are variant stylings of the same term.)
The German Karl Adolph von Basedow independently reported the same constellation of symptoms in 1840.[43][44] As a result, on the European continent, the terms «Basedow syndrome»,[45] «Basedow disease», or «Morbus Basedow»[46] are more common than «Graves’ disease».[45][47]
Graves’ disease[45][46] has also been called exophthalmic goiter.[46]
Less commonly, it has been known as Parry disease,[45][46] Begbie disease, Flajan disease, Flajani–Basedow syndrome, and Marsh disease.[45] These names for the disease were derived from Caleb Hillier Parry, James Begbie, Giuseppe Flajani, and Henry Marsh.[45] Early reports, not widely circulated, of cases of goiter with exophthalmos were published by the Italians Giuseppe Flajani[48] and Antonio Giuseppe Testa,[49] in 1802 and 1810, respectively.[50] Prior to these, Caleb Hillier Parry,[51] a notable provincial physician in England of the late-18th century (and a friend of Edward Miller-Gallus),[52] described a case in 1786. This case was not published until 1825 — ten years ahead of Graves.[53]
However, fair credit for the first description of Graves’ disease goes to the 12th century Persian physician Sayyid Ismail al-Jurjani,[54] who noted the association of goiter and exophthalmos in his Thesaurus of the Shah of Khwarazm, the major medical dictionary of its time.[45][55]
Society and culture[edit]
Notable cases[edit]
Marty Feldman used his bulging eyes, caused by Graves’ disease, for comedic effect.
- Ayaka, Japanese singer, was diagnosed with Graves’ disease in 2007. After going public with her diagnosis in 2009, she took a two-year hiatus from music to focus on treatment.[56][57]
- Susan Elizabeth Blow, American educator and founder of the first publicly funded kindergarten in the United States, was forced to retire and seek treatment for Graves’ disease in 1884.[58]
- George H. W. Bush, former U.S. president, developed new atrial fibrillation and was diagnosed in 1991 with hyperthyroidism due to the disease and treated with radioactive iodine. The president’s wife, Barbara Bush, also developed the disease around the same time, which, in her case, produced severe infiltrative exophthalmos.[59]
- Rodney Dangerfield, American comedian and actor[60]
- Gail Devers, American sprinter: A doctor considered amputating her feet after she developed blistering and swelling following radiation treatment for Graves’ disease, but she went on to recover and win Olympic medals.
- Missy Elliott, American hip-hop artist[61]
- Marty Feldman, British comedy writer, comedian and actor[62][63]
- Sia, Australian singer and songwriter[64]
- Sammy Gravano, Italian-American former underboss of the Gambino crime family.[65]
- Jim Hamilton, Scottish rugby player, discovered he had Graves’ disease shortly after retiring from the sport in 2017.[66]
- Heino, German folk singer, whose dark sunglasses (worn to hide his symptoms) became part of his trademark look[67]
- Herbert Howells, British composer; the first person to be treated with radium injections[68]
- Yayoi Kusama, Japanese artist.[69]
- Nadezhda Krupskaya, Russian Communist and wife of Vladimir Lenin[70]
- Umm Kulthum was an Egyptian singer, songwriter, and film actress active from the 1920s to the 1970s.
- Barbara Leigh, an American former actress and fashion model, now spokeswoman for the National Graves’ Disease Foundation[71]
- Keiko Masuda, Japanese singer and one-half of the duo Pink Lady.[72][73][74][75]
- Yūko Miyamura, Japanese voice actress[76]
- Lord Monckton, former UKIP and Conservative politician.[77]
- Sophia Parnok, Russian poet[78][79][80]
- Sir Cecil Spring Rice, British ambassador to the United States during World War I, died suddenly of the disease in 1918.[81]
- Christina Rossetti, English Victorian-era poet[82]
- Dame Maggie Smith, British actress[83]
- Mary Webb, British novelist and poet[84]
- Wendy Williams, American TV show host[85]
- Act Yasukawa, Japanese Professional wrestler.[86]
Literature[edit]
- In Italo Svevo’s novel Zeno’s Conscience, character Ada develops the disease.[87][88]
- Ern Malley was an acclaimed Australian poet whose work was not published until after his death from Graves’ disease in 1943. However, Malley’s existence and entire biography was actually later revealed to be a literary hoax.
Research[edit]
Agents that act as antagonists at thyroid stimulating hormone receptors are currently under investigation as a possible treatment for Graves’ disease.[89]
References[edit]
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z «Graves Disease». www.niddk.nih.gov. August 10, 2012. Archived from the original on April 2, 2015. Retrieved 2015-04-02.
- ^ «Graves’ disease». Autoimmune Registry Inc. Retrieved 15 June 2022.
- ^ a b c Menconi F, Marcocci C, Marinò M (2014). «Diagnosis and classification of Graves disease». Autoimmunity Reviews. 13 (4–5): 398–402. doi:10.1016/j.autrev.2014.01.013. PMID 24424182.
- ^ a b c d e f g h Brent GA (June 2008). «Clinical practice. Grave disease». The New England Journal of Medicine. 358 (24): 2594–605. doi:10.1056/NEJMcp0801880. PMID 18550875.
- ^ a b c Burch HB, Cooper DS (December 2015). «Management of Graves Disease: A Review». JAMA. 314 (23): 2544–54. doi:10.1001/jama.2015.16535. PMID 26670972.
- ^ a b c d e Nikiforov YE, Biddinger PW, Nikiforova LD, Biddinger PW (2012). Diagnostic pathology and molecular genetics of the thyroid (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 69. ISBN 9781451114553. Archived from the original on 2017-09-08.
- ^ N Burrow G, H Oppenheimer J, Volpé R (1989). Thyroid function & disease. ISBN 0721621902.
- ^ a b page 157 in:Agabegi ED, Agabegi SS (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 978-0-7817-7153-5.
- ^ Bunevicius R, Prange AJ (2006). «Psychiatric manifestations of Graves hyperthyroidism: pathophysiology and treatment options». CNS Drugs. 20 (11): 897–909. doi:10.2165/00023210-200620110-00003. PMID 17044727. S2CID 20003511.
- ^ Falgarone G, Heshmati HM, Cohen R, Reach G (January 2013). «Mechanisms in endocrinology. Role of emotional stress in the pathophysiology of Graves’ disease». European Journal of Endocrinology. 168 (1): R13-8. doi:10.1530/EJE-12-0539. PMID 23027804.
- ^ a b Tomer Y, Davies TF (February 1993). «Infection, thyroid disease, and autoimmunity». Endocrine Reviews. 14 (1): 107–20. doi:10.1210/edrv-14-1-107. PMID 8491150.
- ^ Smith TJ, Hegedüs L (October 2016). «Graves’ Disease» (PDF). The New England Journal of Medicine. 375 (16): 1552–1565. doi:10.1056/NEJMra1510030. PMID 27797318. Archived from the original (PDF) on 2020-08-01. Retrieved 2020-05-29.
- ^ Desailloud R, Hober D (January 2009). «Viruses and thyroiditis: an update». Virology Journal. 6: 5. doi:10.1186/1743-422X-6-5. PMC 2654877. PMID 19138419.
- ^ Toivanen P, Toivanen A (1994). «Does Yersinia induce autoimmunity?». International Archives of Allergy and Immunology. 104 (2): 107–11. doi:10.1159/000236717. PMID 8199453.
- ^ Strieder TG, Wenzel BE, Prummel MF, Tijssen JG, Wiersinga WM (May 2003). «Increased prevalence of antibodies to enteropathogenic Yersinia enterocolitica virulence proteins in relatives of patients with autoimmune thyroid disease». Clinical and Experimental Immunology. 132 (2): 278–82. doi:10.1046/j.1365-2249.2003.02139.x. PMC 1808711. PMID 12699417.
- ^ Hansen PS, Wenzel BE, Brix TH, Hegedüs L (October 2006). «Yersinia enterocolitica infection does not confer an increased risk of thyroid antibodies: evidence from a Danish twin study». Clinical and Experimental Immunology. 146 (1): 32–8. doi:10.1111/j.1365-2249.2006.03183.x. PMC 1809723. PMID 16968395.
- ^ Moore EA, Moore LM (2013). Advances in Graves’ Disease and Other Hyperthyroid Disorders. McFarland. p. 77. ISBN 9780786471898.
- ^ «Thyroid Disease, Osteoporosis and Calcium – Womens Health and Medical Information on». Medicinenet.com. 2006-12-07. Archived from the original on 2013-03-07. Retrieved 2013-02-27.
- ^ a b Carnell NE, Valente WA (July 1998). «Thyroid nodules in Graves’ disease: classification, characterization, and response to treatment». Thyroid. 8 (7): 571–6. doi:10.1089/thy.1998.8.571. PMID 9709909.
- ^ Wallaschofski H, Kuwert T, Lohmann T (April 2004). «TSH-receptor autoantibodies — differentiation of hyperthyroidism between Graves’ disease and toxic multinodular goitre». Experimental and Clinical Endocrinology & Diabetes. 112 (4): 171–4. doi:10.1055/s-2004-817930. PMID 15127319.
- ^ Shan SJ, Douglas RS (June 2014). «The pathophysiology of thyroid eye disease». Journal of Neuro-Ophthalmology. 34 (2): 177–85. doi:10.1097/wno.0000000000000132. PMID 24821101. S2CID 10998666.
- ^ Feldon SE, Muramatsu S, Weiner JM (October 1984). «Clinical classification of Graves’ ophthalmopathy. Identification of risk factors for optic neuropathy». Archives of Ophthalmology. 102 (10): 1469–72. doi:10.1001/archopht.1984.01040031189015. PMID 6548373.
- ^ Gorman CA (June 1998). «The measurement of change in Graves’ ophthalmopathy». Thyroid. 8 (6): 539–43. doi:10.1089/thy.1998.8.539. PMID 9669294.
- ^ Tan NY, Leong YY, Lang SS, Htoon ZM, Young SM, Sundar G (May 2017). «Radiologic Parameters of Orbital Bone Remodeling in Thyroid Eye Disease». Investigative Ophthalmology & Visual Science. 58 (5): 2527–2533. doi:10.1167/iovs.16-21035. PMID 28492870.
- ^ Cawood T, Moriarty P, O’Shea D (August 2004). «Recent developments in thyroid eye disease». BMJ. 329 (7462): 385–90. doi:10.1136/bmj.329.7462.385. PMC 509348. PMID 15310608.
- ^ Bartley GB (March 2011). «Rundle and his curve». Archives of Ophthalmology. 129 (3): 356–8. doi:10.1001/archophthalmol.2011.29. PMID 21402995.
- ^ a b c d e Stathopoulos P, Gangidi S, Kotrotsos G, Cunliffe D (June 2015). «Graves’ disease: a review of surgical indications, management, and complications in a cohort of 59 patients». International Journal of Oral and Maxillofacial Surgery. 44 (6): 713–7. doi:10.1016/j.ijom.2015.02.007. PMID 25726089.
- ^ Homsanit M, Sriussadaporn S, Vannasaeng S, Peerapatdit T, Nitiyanant W, Vichayanrat A (March 2001). «Efficacy of single daily dosage of methimazole vs. propylthiouracil in the induction of euthyroidism». Clinical Endocrinology. 54 (3): 385–90. doi:10.1046/j.1365-2265.2001.01239.x. PMID 11298092. S2CID 24463399.
- ^ Glinoer D, de Nayer P, Bex M (May 2001). «Effects of l-thyroxine administration, TSH-receptor antibodies and smoking on the risk of recurrence in Graves’ hyperthyroidism treated with antithyroid drugs: a double-blind prospective randomized study». European Journal of Endocrinology. 144 (5): 475–83. doi:10.1530/eje.0.1440475. PMID 11331213.
- ^ Saha GB (2009). Fundamentals of Nuclear Pharmacy (5 ed.). Springer-Verlag New York, LLC. p. 342. ISBN 978-0387403601.
- ^ Schäffler A (November 2010). «Hormone replacement after thyroid and parathyroid surgery». Deutsches Ärzteblatt International. 107 (47): 827–34. doi:10.3238/arztebl.2010.0827. PMC 3003466. PMID 21173898.
- ^ a b «Treatment of an Over-active or Enlarged Thyroid Gland with Radioactive Iodine – British Thyroid Foundation». Btf-thyroid.org. Archived from the original on 2016-09-02. Retrieved 2016-09-10.
- ^ Mizokami, Tetsuya; Hamada, Katsuhiko; Maruta, Tetsushi; Higashi, Kiichiro; Tajiri, Junichi (September 2016). «Painful Radiation Thyroiditis after 131I Therapy for Graves’ Hyperthyroidism: Clinical Features and Ultrasonographic Findings in Five Cases». European Thyroid Journal. 5 (3): 201–206. doi:10.1159/000448398. ISSN 2235-0640. PMC 5091234. PMID 27843811.
- ^ Genovese BM, Noureldine SI, Gleeson EM, Tufano RP, Kandil E (February 2013). «What is the best definitive treatment for Graves’ disease? A systematic review of the existing literature». Annals of Surgical Oncology (review). 20 (2): 660–7. doi:10.1245/s10434-012-2606-x. PMID 22956065. S2CID 24759725.
- ^ Limongi, Roberto Murillo; Feijó, Eduardo Damous; Rodrigues Lopes E Silva, Marlos; Akaishi, Patrícia; Velasco E Cruz, Antônio Augusto; Christian Pieroni-Gonçalves, Allan; Pereira, Filipe; Devoto, Martin; Bernardini, Francesco; Marques, Victor; Tao, Jeremiah P. (February 2020). «Orbital Bone Decompression for Non-Thyroid Eye Disease Proptosis». Ophthalmic Plastic and Reconstructive Surgery. 36 (1): 13–16. doi:10.1097/IOP.0000000000001435. ISSN 1537-2677. PMID 31373985. S2CID 199388425.
- ^ «Graves Disease: Overview, Causes, and Symptoms». Healthline. Retrieved 2020-06-08.
- ^ Roy A, Dutta D, Ghosh S, Mukhopadhyay P, Mukhopadhyay S, Chowdhury S (2015). «Efficacy and safety of low dose oral prednisolone as compared to pulse intravenous methylprednisolone in managing moderate severe Graves’ orbitopathy: A randomized controlled trial». Indian Journal of Endocrinology and Metabolism. 19 (3): 351–8. doi:10.4103/2230-8210.152770. PMC 4366772. PMID 25932389.
- ^ a b contributors, ed. Kenneth L. Becker… With 330 (2001). Principles and Practice of Endocrinology and Metabolism (3 ed.). Philadelphia, Pa. [u.a.]: Lippincott, Williams & Wilkins. p. 636. ISBN 9780781717502. Archived from the original on 2017-09-08.
- ^ Carlé, Allan; Pedersen, Inge Bülow; Knudsen, Nils; Perrild, Hans; Ovesen, Lars; Rasmussen, Lone Banke; Laurberg, Peter (2011). «Epidemiology of subtypes of hyperthyroidism in Denmark: a population-based study». European Journal of Endocrinology. 164 (5): 801–809. doi:10.1530/EJE-10-1155. ISSN 0804-4643. PMID 21357288.
- ^ Pokhrel, Binod; Bhusal, Kamal (2020), «Graves Disease», StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 28846288, retrieved 2020-12-04
- ^ Mathew Graves at Who Named It?
- ^ Graves, RJ. Newly observed affection of the thyroid gland in females Archived 2016-03-31 at the Wayback Machine. (Clinical lectures.) London Medical and Surgical Journal (Renshaw), 1835; 7 (part 2): 516–517. Reprinted in Medical Classics, 1940;5:33–36.
- ^ Von Basedow, KA. Exophthalmus durch Hypertrophie des Zellgewebes in der Augenhöhle. [Casper’s] Wochenschrift für die gesammte Heilkunde, Berlin, 1840, 6: 197–204; 220–228. Partial English translation in: Ralph Hermon Major (1884–1970): Classic Descriptions of Disease. Springfield, C. C. Thomas, 1932. 2nd edition, 1939; 3rd edition, 1945.
- ^ Von Basedow, KA. «Die Glotzaugen». [Casper’s] Wochenschrift für die gesammte Heilkunde, Berlin, 1848: 769–777.
- ^ a b c d e f g Basedow syndrome or disease at Who Named It? – the history and naming of the disease
- ^ a b c d Robinson, Victor, ed. (1939). «Exophthalmic goiter, Basedow disease, Grave disesase». The Modern Home Physician, A New Encyclopedia of Medical Knowledge. WM. H. Wise & Company (New York)., pages 82, 294, and 295.
- ^ Goiter, Diffuse Toxic at eMedicine
- ^ Flajani, G. Sopra un tumor freddo nell’anterior parte del collo broncocele. (Osservazione LXVII). In Collezione d’osservazioni e reflessioni di chirurgia. Rome, Michele A Ripa Presso Lino Contedini, 1802;3:270–273.
- ^ Testa, AG. Delle malattie del cuore, loro cagioni, specie, segni e cura. Bologna, 1810. 2nd edition in 3 volumes, Florence, 1823; Milano 1831; German translation, Halle, 1813.
- ^ Giuseppe Flajani at Who Named It?
- ^ Parry CH (1825). «Enlargement of the thyroid gland in connection with enlargement or palpitations of the heart». Collections from the unpublished medical writings of C. H. Parry. London. pp. 111–129.
According to Garrison, Parry first noted the condition in 1786. He briefly reported it in his Elements of Pathology and Therapeutics, 1815. Reprinted in Medical Classics, 1940, 5: 8–30
- ^ Hull G (June 1998). «Caleb Hillier Parry 1755-1822: a notable provincial physician». Journal of the Royal Society of Medicine. 91 (6): 335–8. doi:10.1177/014107689809100618. PMC 1296785. PMID 9771526.
- ^ Caleb Hillier Parry at Who Named It?
- ^ Sayyid Ismail Al-Jurjani. Thesaurus of the Shah of Khwarazm.
- ^ Ljunggren JG (August 1983). «[Who was the man behind the syndrome: Ismail al-Jurjani, Testa, Flagani, Parry, Graves or Basedow? Use the term hyperthyreosis instead]». Läkartidningen. 80 (32–33): 2902. PMID 6355710.
- ^ «水嶋ヒロ・絢香、2ショット会見で結婚報告 絢香はバセドウ病を告白、年内で休業へ» (in Japanese). Oricon. April 3, 2009. Archived from the original on December 8, 2015. Retrieved November 19, 2015.
- ^ «絢香、初のセルフ・プロデュース・アルバムが発売決定!» (in Japanese). CDJournal. December 1, 2011. Archived from the original on October 15, 2015. Retrieved November 19, 2015.
- ^ Shepley, Carol Ferring (2008). Movers and Shakers, Scalawags and Suffragettes: Tales from Bellefontaine Cemetery. St. Louis, Missouri: Missouri History Museum.
- ^ Altman LK (1991-05-28). «The Doctor’s World — A White House Puzzle: Immunity Ailments-Science Section». The New York Times. Archived from the original on 2013-05-08. Retrieved 2013-02-27.
- ^ Islam S (2017-01-23). «Thyroid gland – Hyperplasia / goiter – Graves disease». Pathologyoutlines.com. Archived from the original on 2016-12-14. Retrieved 2017-01-25.
- ^ Oldenburg A (2011-06-24). «Update: Missy Elliott ‘completely managing’ Graves’ disease». USA Today. Gannett.
- ^ «Famous People with Graves’ Disease». HRFnd. December 15, 2013. Retrieved 2018-02-22.
- ^ Kuhlenbeck M (June 29, 2016). «Marty Feldman versus the Suits». Jewish Currents. Retrieved 2018-02-22.
Viewers also could not help being amazed by his bulging eyes, which had resulted from a botched operation for Graves’ disease.
- ^ Rota G. «Facts About Sia Furler | Popsugar Celebrity Australia». Popsugar.com.au. Archived from the original on 2015-02-09. Retrieved 2016-09-10.
- ^ Guart, Al (March 31, 2002). «Rare Disease Could Whack Sammy Bull». New York Post. Archived from the original on January 11, 2012. Retrieved January 28, 2020.
- ^ «Hamilton talks about his disease on his podcast». YouTube. Archived from the original on 2017-09-08.
- ^ «Crossover Crooner: The Strange Comeback of Germany’s Wannabe Johnny Cash». Spiegel.de. 2013-02-07. Archived from the original on 2014-11-19. Retrieved 2014-07-27.
- ^ Spicer P (1998). Herbert Howells. Bridgend: Seren. p. 44. ISBN 1-85411-233-3.
- ^ «Yayoi Kusama by Grady T. Turner». Bomb Magazine. January 1, 1999. Retrieved May 29, 2020.
- ^ «Revolutionary First Lady: the life and struggles of Lenin’s wife». Russia Beyond. Archived from the original on 2018-04-18. Retrieved 2018-04-18.
- ^ «Barbara Leigh». Home.rmci.net. Archived from the original on 2012-07-10. Retrieved 2013-02-27.
- ^ «[歌手 増田恵子さん]バセドー病(1)マイク持つ手が震える». Yomiuri Shimbun. 2011-08-04. Retrieved 2020-02-01.
- ^ «[歌手 増田恵子さん]バセドー病(2)同じ病 姉の存在が支えに». Yomiuri Shimbun. 2011-08-11. Retrieved 2020-02-01.
- ^ «[歌手 増田恵子さん]バセドー病(3)ツアー中、甲状腺腫れ上がる». Yomiuri Shimbun. 2011-08-18. Retrieved 2020-02-01.
- ^ «[歌手 増田恵子さん]バセドー病(4)病気公表 無理せず我慢せず». Yomiuri Shimbun. 2011-08-25. Retrieved 2020-02-01.
- ^ «親子知新». www3.bigcosmic.com. Archived from the original on May 15, 2007. Retrieved December 18, 2017.
- ^ Rupert Murray «Meet the Climate Sceptics» Archived 2013-10-22 at the Wayback Machine, Storyville, 3 February 2011.
- ^ «Sophia Parnok, Russia’s Sappho».
- ^ Burgin, Diana Lewis (1992). «Sophia Parnok and the Writing of a Lesbian Poet’s Life». Slavic Review. 51 (2): 214–231. doi:10.2307/2499528. JSTOR 2499528. S2CID 163967264.
- ^ https://www.king.org/event/the-esoterics-parnok-in-that-infinite-moment/[permanent dead link]
- ^ Simon, Bernard (31 May 2013). «This memorial is poetic justice for Sir Cecil Spring Rice». telegraph.co.uk. Archived from the original on 2014-03-12. Retrieved 2014-08-25.
- ^ «Christina Rossetti». Poetry Foundation. Archived from the original on 2016-04-17. Retrieved 2016-09-10.
- ^ Wolf M (March 18, 1990). «There is Nothing Like This Dame». New York Times. Archived from the original on August 10, 2016. Retrieved 2015-10-19.
- ^ «Biography». Archived from the original on 2015-07-16. Retrieved 2015-07-16.
- ^ Melas C (February 21, 2018). «Wendy Williams announces show hiatus due to Graves’ disease». CNN. Retrieved February 21, 2018.
- ^ «Act Yasukawa Returns To Ring After Five Years Away». 15 November 2020.
- ^ Svevo, Italo (2003). Zeno’s conscience : a novel (1st Vintage International ed.). Vintage Books. pp. 315–321. ISBN 0375727760.
- ^ Scarponi, Mattia (19 August 2017). «Il morbo di Basedow: lo sfinimento tra Zeno e la realtà». theWise Magazine (in Italian). Retrieved 25 March 2020.
- ^ «Thyroid». Mayo Clinic. Archived from the original on 4 November 2016. Retrieved 1 November 2016.
External links[edit]
- «Graves’ disease». Genetics Home Reference. U.S. National Library of Medicine.
- https://www.ncbi.nlm.nih.gov/gene/?term=graves about graves on ncbi
| Graves’ disease | |
|---|---|
| Other names | Toxic diffuse goiter, Flajani–Basedow–Graves disease |
 |
|
| The classic finding of exophthalmos and lid retraction in Graves’ disease | |
| Specialty | Endocrinology |
| Symptoms | Enlarged thyroid, irritability, muscle weakness, sleeping problems, fast heartbeat, weight loss, poor tolerance of heat,[1] anxiety, tremor of hands or fingers, warm and moist skin, increased perspiration, goiter, changes in menstrual cycle, easy bruising, erectile dysfunction, reduced libido, frequent bowel movements, bulging eyes (Graves’ ophthalmopathy), thick red skin on shins or the top of foot (pretibial myxedema)[2] |
| Complications | Graves’ ophthalmopathy[1] |
| Causes | Unknown[3] |
| Risk factors | Family history, other autoimmune diseases[1] |
| Diagnostic method | Blood tests, radioiodine uptake[1][4] |
| Treatment | Radioiodine therapy, medications, thyroid surgery[1] |
| Frequency | 0.5% (males), 3% (females)[5] |
Graves’ disease (German: Morbus Basedow), also known as toxic diffuse goiter, is an autoimmune disease that affects the thyroid.[1] It frequently results in and is the most common cause of hyperthyroidism.[5] It also often results in an enlarged thyroid.[1] Signs and symptoms of hyperthyroidism may include irritability, muscle weakness, sleeping problems, a fast heartbeat, poor tolerance of heat, diarrhea and unintentional weight loss.[1] Other symptoms may include thickening of the skin on the shins, known as pretibial myxedema, and eye bulging, a condition caused by Graves’ ophthalmopathy.[1] About 25 to 30% of people with the condition develop eye problems.[1][4]
The exact cause of the disease is unclear; however, it is believed to involve a combination of genetic and environmental factors.[3] A person is more likely to be affected if they have a family member with the disease.[1] If one twin is affected, a 30% chance exists that the other twin will also have the disease.[6] The onset of disease may be triggered by physical or emotional stress, infection, or giving birth.[4] Those with other autoimmune diseases such as type 1 diabetes and rheumatoid arthritis are more likely to be affected.[1] Smoking increases the risk of disease and may worsen eye problems.[1] The disorder results from an antibody, called thyroid-stimulating immunoglobulin (TSI), that has a similar effect to thyroid stimulating hormone (TSH).[1] These TSI antibodies cause the thyroid gland to produce excess thyroid hormones.[1] The diagnosis may be suspected based on symptoms and confirmed with blood tests and radioiodine uptake.[1][4] Typically, blood tests show a raised T3 and T4, low TSH, increased radioiodine uptake in all areas of the thyroid and TSI antibodies.[4]
The three treatment options are radioiodine therapy, medications, and thyroid surgery.[1] Radioiodine therapy involves taking iodine-131 by mouth, which is then concentrated in the thyroid and destroys it over weeks to months.[1] The resulting hypothyroidism is treated with synthetic thyroid hormones.[1] Medications such as beta blockers may control some of the symptoms, and antithyroid medications such as methimazole may temporarily help people while other treatments are having effect.[1] Surgery to remove the thyroid is another option.[1] Eye problems may require additional treatments.[1]
Graves’ disease will develop in about 0.5% of males and 3% of females.[5] It occurs about 7.5 times more often in women than in men.[1] Often, it starts between the ages of 40 and 60 but can begin at any age.[6] It is the most common cause of hyperthyroidism in the United States (about 50 to 80% of cases).[1][4] The condition is named after Irish surgeon Robert Graves, who described it in 1835.[6] A number of prior descriptions also exist.[6]
Signs and symptoms[edit]
The signs and symptoms of Graves’ disease virtually all result from the direct and indirect effects of hyperthyroidism, with main exceptions being Graves’ ophthalmopathy, goiter, and pretibial myxedema (which are caused by the autoimmune processes of the disease). Symptoms of the resultant hyperthyroidism are mainly insomnia, hand tremor, hyperactivity, hair loss, excessive sweating, oligomenorrhea, itching, heat intolerance, weight loss despite increased appetite, diarrhea, frequent defecation, palpitations, periodic partial muscle weakness or paralysis in those especially of Asian descent,[7] and skin warmth and moistness.[8] Further signs that may be seen on physical examination are most commonly a diffusely enlarged (usually symmetric), nontender thyroid, lid lag, excessive lacrimation due to Graves’ ophthalmopathy, arrhythmias of the heart, such as sinus tachycardia, atrial fibrillation, and premature ventricular contractions, and hypertension.[8][9]
Cause[edit]
The exact cause is unclear; however, it is believed to involve a combination of genetic and environmental factors.[3] While a theoretical mechanism occurs by which exposure to severe stressors and high levels of subsequent distress such as PTSD (Post traumatic stress disorder) could increase the risk of immune disease and cause an aggravation of the autoimmune response that leads to Graves’ disease, more robust clinical data are needed for a firm conclusion.[10]
Genetics[edit]
A genetic predisposition for Graves’ disease is seen, with some people more prone to develop TSH receptor activating antibodies due to a genetic cause. Human leukocyte antigen DR (especially DR3) appears to play a role.[11] To date, no clear genetic defect has been found to point to a single-gene cause.[citation needed]
Genes believed to be involved include those for thyroglobulin, thyrotropin receptor, protein tyrosine phosphatase nonreceptor type 22 (PTPN22), and cytotoxic T-lymphocyte–associated antigen 4, among others.[12]
Infectious trigger[edit]
Since Graves’ disease is an autoimmune disease which appears suddenly, often later in life, a viral or bacterial infection may trigger antibodies which cross-react with the human TSH receptor, a phenomenon known as antigenic mimicry.[13]
The bacterium Yersinia enterocolitica bears structural similarity with the human thyrotropin receptor[11] and was hypothesized to contribute to the development of thyroid autoimmunity arising for other reasons in genetically susceptible individuals.[14]
In the 1990s, it was suggested that Y. enterocolitica may be associated with Graves’ disease.[15]
More recently, the role for Y. enterocolitica has been disputed.[16]
Epstein–Barr virus (EBV) is another potential trigger.[17]
Mechanism[edit]
Thyroid-stimulating immunoglobulins recognize and bind to the thyrotropin receptor (TSH receptor) which stimulates the secretion of thyroxine (T4) and triiodothyronine (T3). Thyroxine receptors in the pituitary gland are activated by the surplus hormone, suppressing additional release of TSH in a negative feedback loop. The result is very high levels of circulating thyroid hormones and a low TSH level.[citation needed]
Pathophysiology[edit]
Histopathological image of diffuse hyperplasia of the thyroid gland (clinically presenting as hyperthyroidism)
Graves’ disease is an autoimmune disorder, in which the body produces antibodies that are specific to a self-protein: the receptor for thyroid-stimulating hormone. (Antibodies to thyroglobulin and to the thyroid hormones T3 and T4 may also be produced.)
These antibodies cause hyperthyroidism because they bind to the TSHr and chronically stimulate it. The TSHr is expressed on the thyroid follicular cells of the thyroid gland (the cells that produce thyroid hormone), and the result of chronic stimulation is an abnormally high production of T3 and T4. This, in turn, causes the clinical symptoms of hyperthyroidism, and the enlargement of the thyroid gland visible as goiter.
The infiltrative exophthalmos frequently encountered has been explained by postulating that the thyroid gland and the extraocular muscles share a common antigen which is recognized by the antibodies. Antibodies binding to the extraocular muscles would cause swelling behind the eyeball.
The «orange peel» skin has been explained by the infiltration of antibodies under the skin, causing an inflammatory reaction and subsequent fibrous plaques.
The three types of autoantibodies to the TSH receptor currently recognized are:
- Thyroid stimulating immunoglobulins: these antibodies (mainly IgG) act as long-acting thyroid stimulants (LATS), activating the cells through a slower and more drawn out process compared to TSH, leading to an elevated production of thyroid hormone.
- Thyroid growth immunoglobulins: these antibodies bind directly to the TSH receptor and have been implicated in the growth of thyroid follicles.
- Thyrotrophin binding-inhibiting immunoglobulins: these antibodies inhibit the normal union of TSH with its receptor.
- Some actually act as if TSH itself is binding to its receptor, thus inducing thyroid function.
- Other types may not stimulate the thyroid gland, but prevent TSI and TSH from binding to and stimulating the receptor.
Another effect of hyperthyroidism is bone loss from osteoporosis, caused by an increased excretion of calcium and phosphorus in the urine and stool. The effects can be minimized if the hyperthyroidism is treated early. Thyrotoxicosis can also augment calcium levels in the blood by as much as 25%. This can cause stomach upset, excessive urination, and impaired kidney function.[18]
Diagnosis[edit]
Graves’ disease may present clinically with one or more of these characteristic signs:[citation needed]
- Rapid heartbeat (80%)
- Diffuse palpable goiter with audible bruit (70%)
- Tremor (40%)
- Exophthalmos (protuberance of one or both eyes), periorbital edema (25%)
- Fatigue (70%), weight loss (60%) with increased appetite in young people and poor appetite in the elderly, and other symptoms of hyperthyroidism/thyrotoxicosis
- Heat intolerance (55%)
- Tremulousness (55%)
- Palpitations (50%)
Two signs are truly ‘diagnostic’ of Graves’ disease (i.e., not seen in other hyperthyroid conditions): exophthalmos and nonpitting edema (pretibial myxedema). Goiter is an enlarged thyroid gland and is of the diffuse type (i.e., spread throughout the gland). Diffuse goiter may be seen with other causes of hyperthyroidism, although Graves’ disease is the most common cause of diffuse goiter. A large goiter will be visible to the naked eye, but a small one (mild enlargement of the gland) may be detectable only by physical examination. Occasionally, goiter is not clinically detectable, but may be seen only with computed tomography or ultrasound examination of the thyroid.[citation needed]
Another sign of Graves’ disease is hyperthyroidism; that is, overproduction of the thyroid hormones T3 and T4. Normal thyroid levels are also seen, and occasionally also hypothyroidism, which may assist in causing goiter (though it is not the cause of the Graves’ disease). Hyperthyroidism in Graves’ disease is confirmed, as with any other cause of hyperthyroidism, by measuring elevated blood levels of free (unbound) T3 and T4.[citation needed]
Other useful laboratory measurements in Graves’ disease include thyroid-stimulating hormone (TSH, usually undetectable in Graves’ disease due to negative feedback from the elevated T3 and T4), and protein-bound iodine (elevated). Serologically detected thyroid-stimulating antibodies, radioactive iodine (RAI) uptake, or thyroid ultrasound with Doppler all can independently confirm a diagnosis of Graves’ disease.
Biopsy to obtain histiological testing is not normally required, but may be obtained if thyroidectomy is performed.
The goiter in Graves’ disease is often not nodular, but thyroid nodules are also common.[19] Differentiating common forms of hyperthyroidism such as Graves’ disease, single thyroid adenoma, and toxic multinodular goiter is important to determine proper treatment.[19] The differentiation among these entities has advanced, as imaging and biochemical tests have improved. Measuring TSH-receptor antibodies with the h-TBII assay has been proven efficient and was the most practical approach found in one study.[20]
Eye disease[edit]
Thyroid-associated ophthalmopathy (TAO), or thyroid eye disease (TED), is the most common extrathyroidal manifestation of Graves’ disease. It is a form of idiopathic lymphocytic orbital inflammation, and although its pathogenesis is not completely understood, autoimmune activation of orbital fibroblasts, which in TAO express the TSH receptor, is thought to play a central role.[21]
Hypertrophy of the extraocular muscles, adipogenesis, and deposition of nonsulfated glycosaminoglycans and hyaluronate, causes expansion of the orbital fat and muscle compartments, which within the confines of the bony orbit may lead to dysthyroid optic neuropathy, increased intraocular pressures, proptosis, venous congestion leading to chemosis and periorbital edema, and progressive remodeling of the orbital walls.[22][23][24] Other distinctive features of TAO include lid retraction, restrictive myopathy, superior limbic keratoconjunctivitis, and exposure keratopathy.[citation needed]
Severity of eye disease may be classified by the mnemonic: «NO SPECS»:[25]
- Class 0: No signs or symptoms
- Class 1: Only signs (limited to upper lid retraction and stare, with or without lid lag)
- Class 2: Soft tissue involvement (oedema of conjunctivae and lids, conjunctival injection, etc.)
- Class 3: Proptosis
- Class 4: Extraocular muscle involvement (usually with diplopia)
- Class 5: Corneal involvement (primarily due to lagophthalmos)
- Class 6: Sight loss (due to optic nerve involvement)
Typically the natural history of TAO follows Rundle’s curve, which describes a rapid worsening during an initial phase, up to a peak of maximum severity, and then improvement to a static plateau without, however, resolving back to a normal condition.[26]
Management[edit]
Treatment of Graves’ disease includes antithyroid drugs that reduce the production of thyroid hormone, radioiodine (radioactive iodine I-131) and thyroidectomy (surgical excision of the gland). As operating on a hyperthyroid patient is dangerous, prior to thyroidectomy, preoperative treatment with antithyroid drugs is given to render the patient euthyroid. Each of these treatments has advantages and disadvantages, and no single treatment approach is considered the best for everyone.[citation needed]
Treatment with antithyroid medications must be administered for six months to two years to be effective. Even then, upon cessation of the drugs, the hyperthyroid state may recur. The risk of recurrence is about 40–50%, and lifelong treatment with antithyroid drugs carries some side effects such as agranulocytosis and liver disease.[27] Side effects of the antithyroid medications include a potentially fatal reduction in the level of white blood cells. Therapy with radioiodine is the most common treatment in the United States, while antithyroid drugs and/or thyroidectomy are used more often in Europe, Japan, and most of the rest of the world.
β-Blockers (such as propranolol) may be used to inhibit the sympathetic nervous system symptoms of tachycardia and nausea until antithyroid treatments start to take effect. Pure β-blockers do not inhibit lid retraction in the eyes, which is mediated by alpha adrenergic receptors.
Antithyroid drugs[edit]
The main antithyroid drugs are carbimazole (in the UK), methimazole (in the US), and propylthiouracil/PTU. These drugs block the binding of iodine and coupling of iodotyrosines. The most dangerous side effect is agranulocytosis (1/250, more in PTU). Others include granulocytopenia (dose-dependent, which improves on cessation of the drug) and aplastic anemia. Patients on these medications should see a doctor if they develop sore throat or fever. The most common side effects are rash and peripheral neuritis. These drugs also cross the placenta and are secreted in breast milk. Lugol’s iodine may be used to block hormone synthesis before surgery.[citation needed]
A randomized control trial testing single-dose treatment for Graves’ found methimazole achieved euthyroid state more effectively after 12 weeks than did propylthyouracil (77.1% on methimazole 15 mg vs 19.4% in the propylthiouracil 150 mg groups).[28]
No difference in outcome was shown for adding thyroxine to antithyroid medication and continuing thyroxine versus placebo after antithyroid medication withdrawal. However, two markers were found that can help predict the risk of recurrence. These two markers are a positive TSHr antibody (TSHR-Ab) and smoking. A positive TSHR-Ab at the end of antithyroid drug treatment increases the risk of recurrence to 90% (sensitivity 39%, specificity 98%), and a negative TSHR-Ab at the end of antithyroid drug treatment is associated with a 78% chance of remaining in remission. Smoking was shown to have an impact independent to a positive TSHR-Ab.[29]
Radioiodine[edit]
Scan of affected thyroid before (top) and after (bottom) radioiodine therapy
Radioiodine (radioactive iodine-131) was developed in the early 1940s at the Mallinckrodt General Clinical Research Center. This modality is suitable for most patients, although some prefer to use it mainly for older patients. Indications for radioiodine are failed medical therapy or surgery and where medical or surgical therapy are contraindicated. Hypothyroidism may be a complication of this therapy, but may be treated with thyroid hormones if it appears. The rationale for radioactive iodine is that it accumulates in the thyroid and irradiates the gland with its beta and gamma radiations, about 90% of the total radiation being emitted by the beta (electron) particles. The most common method of iodine-131 treatment is to administer a specified amount in microcuries per gram of thyroid gland based on palpation or radiodiagnostic imaging of the gland over 24 hours.[30] Patients who receive the therapy must be monitored regularly with thyroid blood tests to ensure they are treated with thyroid hormone before they become symptomatically hypothyroid.[31]
Contraindications to RAI are pregnancy (absolute), ophthalmopathy (relative; it can aggravate thyroid eye disease), or solitary nodules.[32]
Disadvantages of this treatment are a high incidence of hypothyroidism (up to 80%) requiring eventual thyroid hormone supplementation in the form of a daily pill(s). The radioiodine treatment acts slowly (over months to years) to destroy the thyroid gland, and Graves’ disease–associated hyperthyroidism is not cured in all persons by radioiodine, but has a relapse rate that depends on the dose of radioiodine which is administered.[32] In rare cases, radiation induced thyroiditis has been linked to this treatment.[33]
Surgery[edit]
This modality is suitable for young and pregnant people. Indications for thyroidectomy can be separated into absolute indications or relative indications. These indications aid in deciding which people would benefit most from surgery.[27] The absolute indications are a large goiter (especially when compressing the trachea), suspicious nodules or suspected cancer (to pathologically examine the thyroid), and people with ophthalmopathy and additionally if it is the person’s preferred method of treatment or if refusing to undergo radioactive iodine treatment. Pregnancy is advised to be delayed for 6 months after radioactive iodine treatment.[27]
Both bilateral subtotal thyroidectomy and the Hartley-Dunhill procedure (hemithyroidectomy on one side and partial lobectomy on other side) are possible.
Advantages are immediate cure and potential removal of carcinoma. Its risks are injury of the recurrent laryngeal nerve, hypoparathyroidism (due to removal of the parathyroid glands), hematoma (which can be life-threatening if it compresses the trachea), relapse following medical treatment, infections (less common), and scarring.[27] The increase in the risk of nerve injury can be due to the increased vascularity of the thyroid parenchyma and the development of links between the thyroid capsule and the surrounding tissues. Reportedly, a 1% incidence exists of permanent recurrent laryngeal nerve paralysis after complete thyroidectomy.[27] Risks related to anesthesia are many, thus coordination with the anesthesiologist and patient optimization for surgery preoperatively are essential. Removal of the gland enables complete biopsy to be performed to have definite evidence of cancer anywhere in the thyroid. (Needle biopsies are not so accurate at predicting a benign state of the thyroid). No further treatment of the thyroid is required, unless cancer is detected. Radioiodine uptake study may be done after surgery, to ensure all remaining (potentially cancerous) thyroid cells (i.e., near the nerves to the vocal cords) are destroyed. Besides this, the only remaining treatment will be levothyroxine, or thyroid replacement pills to be taken for the rest of the patient’s life.
A 2013 review article concludes that surgery appears to be the most successful in the management of Graves’ disease, with total thyroidectomy being the preferred surgical option.[34]
Eyes[edit]
Mild cases are treated with lubricant eye drops or nonsteroidal anti-inflammatory drops. Severe cases threatening vision (corneal exposure or optic nerve compression) are treated with steroids or orbital decompression. In all cases, cessation of smoking is essential. Double vision can be corrected with prism glasses and surgery (the latter only when the process has been stable for a while).
Difficulty closing eyes can be treated with lubricant gel at night, or with tape on the eyes to enable full, deep sleep.
Orbital decompression can be performed to enable bulging eyes to retreat back into the head. Bone is removed from the skull behind the eyes, and space is made for the muscles and fatty tissue to fall back into the skull. [35]
Eyelid surgery can be performed on upper and/or lower eyelids to reverse the effects of Graves’ disease[36] on the eyelids. Eyelid muscles can become tight with Graves’ disease, making it impossible to close the eyes all the way. Eyelid surgery involves an incision along the natural crease of the eyelid, and a scraping away of the muscle that holds the eyelid open. This makes the muscle weaker, which allows the eyelid to extend over the eyeball more effectively. Eyelid surgery helps reduce or eliminate dry eye symptoms.
For management of clinically active Graves’ disease, orbitopathy (clinical activity score >2) with at least mild to moderate severity, intravenous glucocorticoids are the treatment of choice, usually administered in the form of pulse intravenous methylprednisolone. Studies have consistently shown that pulse intravenous methylprednisolone is superior to oral glucocorticoids both in terms of efficacy and decreased side effects for managing Graves’ orbitopathy.[37]
Prognosis[edit]
If left untreated, more serious complications could result, including birth defects in pregnancy, increased risk of a miscarriage, bone mineral loss[38] and, in extreme cases, death. Graves’ disease is often accompanied by an increase in heart rate, which may lead to further heart complications, including loss of the normal heart rhythm (atrial fibrillation), which may lead to stroke. If the eyes are proptotic (bulging) enough that the lids do not close completely at night, dryness will occur – with the risk of a secondary corneal infection, which could lead to blindness. Pressure on the optic nerve behind the globe can lead to visual field defects and vision loss, as well. Prolonged untreated hyperthyroidism can lead to bone loss, which may resolve when treated.[38]
Epidemiology[edit]
Graves’ disease occurs in about 0.5% of people.[4] Graves’ disease data has shown that the lifetime risk for women is around 3% and 0.5% for men.[40] It occurs about 7.5 times more often in women than in men[1] and often starts between the ages of 40 and 60.[6] It is the most common cause of hyperthyroidism in the United States (about 50 to 80% of cases).[1][4]
History[edit]
Graves’ disease owes its name to the Irish doctor Robert James Graves,[41] who described a case of goiter with exophthalmos in 1835.[42] (Medical eponyms are often styled nonpossessively; thus Graves’ disease and Graves disease are variant stylings of the same term.)
The German Karl Adolph von Basedow independently reported the same constellation of symptoms in 1840.[43][44] As a result, on the European continent, the terms «Basedow syndrome»,[45] «Basedow disease», or «Morbus Basedow»[46] are more common than «Graves’ disease».[45][47]
Graves’ disease[45][46] has also been called exophthalmic goiter.[46]
Less commonly, it has been known as Parry disease,[45][46] Begbie disease, Flajan disease, Flajani–Basedow syndrome, and Marsh disease.[45] These names for the disease were derived from Caleb Hillier Parry, James Begbie, Giuseppe Flajani, and Henry Marsh.[45] Early reports, not widely circulated, of cases of goiter with exophthalmos were published by the Italians Giuseppe Flajani[48] and Antonio Giuseppe Testa,[49] in 1802 and 1810, respectively.[50] Prior to these, Caleb Hillier Parry,[51] a notable provincial physician in England of the late-18th century (and a friend of Edward Miller-Gallus),[52] described a case in 1786. This case was not published until 1825 — ten years ahead of Graves.[53]
However, fair credit for the first description of Graves’ disease goes to the 12th century Persian physician Sayyid Ismail al-Jurjani,[54] who noted the association of goiter and exophthalmos in his Thesaurus of the Shah of Khwarazm, the major medical dictionary of its time.[45][55]
Society and culture[edit]
Notable cases[edit]
Marty Feldman used his bulging eyes, caused by Graves’ disease, for comedic effect.
- Ayaka, Japanese singer, was diagnosed with Graves’ disease in 2007. After going public with her diagnosis in 2009, she took a two-year hiatus from music to focus on treatment.[56][57]
- Susan Elizabeth Blow, American educator and founder of the first publicly funded kindergarten in the United States, was forced to retire and seek treatment for Graves’ disease in 1884.[58]
- George H. W. Bush, former U.S. president, developed new atrial fibrillation and was diagnosed in 1991 with hyperthyroidism due to the disease and treated with radioactive iodine. The president’s wife, Barbara Bush, also developed the disease around the same time, which, in her case, produced severe infiltrative exophthalmos.[59]
- Rodney Dangerfield, American comedian and actor[60]
- Gail Devers, American sprinter: A doctor considered amputating her feet after she developed blistering and swelling following radiation treatment for Graves’ disease, but she went on to recover and win Olympic medals.
- Missy Elliott, American hip-hop artist[61]
- Marty Feldman, British comedy writer, comedian and actor[62][63]
- Sia, Australian singer and songwriter[64]
- Sammy Gravano, Italian-American former underboss of the Gambino crime family.[65]
- Jim Hamilton, Scottish rugby player, discovered he had Graves’ disease shortly after retiring from the sport in 2017.[66]
- Heino, German folk singer, whose dark sunglasses (worn to hide his symptoms) became part of his trademark look[67]
- Herbert Howells, British composer; the first person to be treated with radium injections[68]
- Yayoi Kusama, Japanese artist.[69]
- Nadezhda Krupskaya, Russian Communist and wife of Vladimir Lenin[70]
- Umm Kulthum was an Egyptian singer, songwriter, and film actress active from the 1920s to the 1970s.
- Barbara Leigh, an American former actress and fashion model, now spokeswoman for the National Graves’ Disease Foundation[71]
- Keiko Masuda, Japanese singer and one-half of the duo Pink Lady.[72][73][74][75]
- Yūko Miyamura, Japanese voice actress[76]
- Lord Monckton, former UKIP and Conservative politician.[77]
- Sophia Parnok, Russian poet[78][79][80]
- Sir Cecil Spring Rice, British ambassador to the United States during World War I, died suddenly of the disease in 1918.[81]
- Christina Rossetti, English Victorian-era poet[82]
- Dame Maggie Smith, British actress[83]
- Mary Webb, British novelist and poet[84]
- Wendy Williams, American TV show host[85]
- Act Yasukawa, Japanese Professional wrestler.[86]
Literature[edit]
- In Italo Svevo’s novel Zeno’s Conscience, character Ada develops the disease.[87][88]
- Ern Malley was an acclaimed Australian poet whose work was not published until after his death from Graves’ disease in 1943. However, Malley’s existence and entire biography was actually later revealed to be a literary hoax.
Research[edit]
Agents that act as antagonists at thyroid stimulating hormone receptors are currently under investigation as a possible treatment for Graves’ disease.[89]
References[edit]
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z «Graves Disease». www.niddk.nih.gov. August 10, 2012. Archived from the original on April 2, 2015. Retrieved 2015-04-02.
- ^ «Graves’ disease». Autoimmune Registry Inc. Retrieved 15 June 2022.
- ^ a b c Menconi F, Marcocci C, Marinò M (2014). «Diagnosis and classification of Graves disease». Autoimmunity Reviews. 13 (4–5): 398–402. doi:10.1016/j.autrev.2014.01.013. PMID 24424182.
- ^ a b c d e f g h Brent GA (June 2008). «Clinical practice. Grave disease». The New England Journal of Medicine. 358 (24): 2594–605. doi:10.1056/NEJMcp0801880. PMID 18550875.
- ^ a b c Burch HB, Cooper DS (December 2015). «Management of Graves Disease: A Review». JAMA. 314 (23): 2544–54. doi:10.1001/jama.2015.16535. PMID 26670972.
- ^ a b c d e Nikiforov YE, Biddinger PW, Nikiforova LD, Biddinger PW (2012). Diagnostic pathology and molecular genetics of the thyroid (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 69. ISBN 9781451114553. Archived from the original on 2017-09-08.
- ^ N Burrow G, H Oppenheimer J, Volpé R (1989). Thyroid function & disease. ISBN 0721621902.
- ^ a b page 157 in:Agabegi ED, Agabegi SS (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 978-0-7817-7153-5.
- ^ Bunevicius R, Prange AJ (2006). «Psychiatric manifestations of Graves hyperthyroidism: pathophysiology and treatment options». CNS Drugs. 20 (11): 897–909. doi:10.2165/00023210-200620110-00003. PMID 17044727. S2CID 20003511.
- ^ Falgarone G, Heshmati HM, Cohen R, Reach G (January 2013). «Mechanisms in endocrinology. Role of emotional stress in the pathophysiology of Graves’ disease». European Journal of Endocrinology. 168 (1): R13-8. doi:10.1530/EJE-12-0539. PMID 23027804.
- ^ a b Tomer Y, Davies TF (February 1993). «Infection, thyroid disease, and autoimmunity». Endocrine Reviews. 14 (1): 107–20. doi:10.1210/edrv-14-1-107. PMID 8491150.
- ^ Smith TJ, Hegedüs L (October 2016). «Graves’ Disease» (PDF). The New England Journal of Medicine. 375 (16): 1552–1565. doi:10.1056/NEJMra1510030. PMID 27797318. Archived from the original (PDF) on 2020-08-01. Retrieved 2020-05-29.
- ^ Desailloud R, Hober D (January 2009). «Viruses and thyroiditis: an update». Virology Journal. 6: 5. doi:10.1186/1743-422X-6-5. PMC 2654877. PMID 19138419.
- ^ Toivanen P, Toivanen A (1994). «Does Yersinia induce autoimmunity?». International Archives of Allergy and Immunology. 104 (2): 107–11. doi:10.1159/000236717. PMID 8199453.
- ^ Strieder TG, Wenzel BE, Prummel MF, Tijssen JG, Wiersinga WM (May 2003). «Increased prevalence of antibodies to enteropathogenic Yersinia enterocolitica virulence proteins in relatives of patients with autoimmune thyroid disease». Clinical and Experimental Immunology. 132 (2): 278–82. doi:10.1046/j.1365-2249.2003.02139.x. PMC 1808711. PMID 12699417.
- ^ Hansen PS, Wenzel BE, Brix TH, Hegedüs L (October 2006). «Yersinia enterocolitica infection does not confer an increased risk of thyroid antibodies: evidence from a Danish twin study». Clinical and Experimental Immunology. 146 (1): 32–8. doi:10.1111/j.1365-2249.2006.03183.x. PMC 1809723. PMID 16968395.
- ^ Moore EA, Moore LM (2013). Advances in Graves’ Disease and Other Hyperthyroid Disorders. McFarland. p. 77. ISBN 9780786471898.
- ^ «Thyroid Disease, Osteoporosis and Calcium – Womens Health and Medical Information on». Medicinenet.com. 2006-12-07. Archived from the original on 2013-03-07. Retrieved 2013-02-27.
- ^ a b Carnell NE, Valente WA (July 1998). «Thyroid nodules in Graves’ disease: classification, characterization, and response to treatment». Thyroid. 8 (7): 571–6. doi:10.1089/thy.1998.8.571. PMID 9709909.
- ^ Wallaschofski H, Kuwert T, Lohmann T (April 2004). «TSH-receptor autoantibodies — differentiation of hyperthyroidism between Graves’ disease and toxic multinodular goitre». Experimental and Clinical Endocrinology & Diabetes. 112 (4): 171–4. doi:10.1055/s-2004-817930. PMID 15127319.
- ^ Shan SJ, Douglas RS (June 2014). «The pathophysiology of thyroid eye disease». Journal of Neuro-Ophthalmology. 34 (2): 177–85. doi:10.1097/wno.0000000000000132. PMID 24821101. S2CID 10998666.
- ^ Feldon SE, Muramatsu S, Weiner JM (October 1984). «Clinical classification of Graves’ ophthalmopathy. Identification of risk factors for optic neuropathy». Archives of Ophthalmology. 102 (10): 1469–72. doi:10.1001/archopht.1984.01040031189015. PMID 6548373.
- ^ Gorman CA (June 1998). «The measurement of change in Graves’ ophthalmopathy». Thyroid. 8 (6): 539–43. doi:10.1089/thy.1998.8.539. PMID 9669294.
- ^ Tan NY, Leong YY, Lang SS, Htoon ZM, Young SM, Sundar G (May 2017). «Radiologic Parameters of Orbital Bone Remodeling in Thyroid Eye Disease». Investigative Ophthalmology & Visual Science. 58 (5): 2527–2533. doi:10.1167/iovs.16-21035. PMID 28492870.
- ^ Cawood T, Moriarty P, O’Shea D (August 2004). «Recent developments in thyroid eye disease». BMJ. 329 (7462): 385–90. doi:10.1136/bmj.329.7462.385. PMC 509348. PMID 15310608.
- ^ Bartley GB (March 2011). «Rundle and his curve». Archives of Ophthalmology. 129 (3): 356–8. doi:10.1001/archophthalmol.2011.29. PMID 21402995.
- ^ a b c d e Stathopoulos P, Gangidi S, Kotrotsos G, Cunliffe D (June 2015). «Graves’ disease: a review of surgical indications, management, and complications in a cohort of 59 patients». International Journal of Oral and Maxillofacial Surgery. 44 (6): 713–7. doi:10.1016/j.ijom.2015.02.007. PMID 25726089.
- ^ Homsanit M, Sriussadaporn S, Vannasaeng S, Peerapatdit T, Nitiyanant W, Vichayanrat A (March 2001). «Efficacy of single daily dosage of methimazole vs. propylthiouracil in the induction of euthyroidism». Clinical Endocrinology. 54 (3): 385–90. doi:10.1046/j.1365-2265.2001.01239.x. PMID 11298092. S2CID 24463399.
- ^ Glinoer D, de Nayer P, Bex M (May 2001). «Effects of l-thyroxine administration, TSH-receptor antibodies and smoking on the risk of recurrence in Graves’ hyperthyroidism treated with antithyroid drugs: a double-blind prospective randomized study». European Journal of Endocrinology. 144 (5): 475–83. doi:10.1530/eje.0.1440475. PMID 11331213.
- ^ Saha GB (2009). Fundamentals of Nuclear Pharmacy (5 ed.). Springer-Verlag New York, LLC. p. 342. ISBN 978-0387403601.
- ^ Schäffler A (November 2010). «Hormone replacement after thyroid and parathyroid surgery». Deutsches Ärzteblatt International. 107 (47): 827–34. doi:10.3238/arztebl.2010.0827. PMC 3003466. PMID 21173898.
- ^ a b «Treatment of an Over-active or Enlarged Thyroid Gland with Radioactive Iodine – British Thyroid Foundation». Btf-thyroid.org. Archived from the original on 2016-09-02. Retrieved 2016-09-10.
- ^ Mizokami, Tetsuya; Hamada, Katsuhiko; Maruta, Tetsushi; Higashi, Kiichiro; Tajiri, Junichi (September 2016). «Painful Radiation Thyroiditis after 131I Therapy for Graves’ Hyperthyroidism: Clinical Features and Ultrasonographic Findings in Five Cases». European Thyroid Journal. 5 (3): 201–206. doi:10.1159/000448398. ISSN 2235-0640. PMC 5091234. PMID 27843811.
- ^ Genovese BM, Noureldine SI, Gleeson EM, Tufano RP, Kandil E (February 2013). «What is the best definitive treatment for Graves’ disease? A systematic review of the existing literature». Annals of Surgical Oncology (review). 20 (2): 660–7. doi:10.1245/s10434-012-2606-x. PMID 22956065. S2CID 24759725.
- ^ Limongi, Roberto Murillo; Feijó, Eduardo Damous; Rodrigues Lopes E Silva, Marlos; Akaishi, Patrícia; Velasco E Cruz, Antônio Augusto; Christian Pieroni-Gonçalves, Allan; Pereira, Filipe; Devoto, Martin; Bernardini, Francesco; Marques, Victor; Tao, Jeremiah P. (February 2020). «Orbital Bone Decompression for Non-Thyroid Eye Disease Proptosis». Ophthalmic Plastic and Reconstructive Surgery. 36 (1): 13–16. doi:10.1097/IOP.0000000000001435. ISSN 1537-2677. PMID 31373985. S2CID 199388425.
- ^ «Graves Disease: Overview, Causes, and Symptoms». Healthline. Retrieved 2020-06-08.
- ^ Roy A, Dutta D, Ghosh S, Mukhopadhyay P, Mukhopadhyay S, Chowdhury S (2015). «Efficacy and safety of low dose oral prednisolone as compared to pulse intravenous methylprednisolone in managing moderate severe Graves’ orbitopathy: A randomized controlled trial». Indian Journal of Endocrinology and Metabolism. 19 (3): 351–8. doi:10.4103/2230-8210.152770. PMC 4366772. PMID 25932389.
- ^ a b contributors, ed. Kenneth L. Becker… With 330 (2001). Principles and Practice of Endocrinology and Metabolism (3 ed.). Philadelphia, Pa. [u.a.]: Lippincott, Williams & Wilkins. p. 636. ISBN 9780781717502. Archived from the original on 2017-09-08.
- ^ Carlé, Allan; Pedersen, Inge Bülow; Knudsen, Nils; Perrild, Hans; Ovesen, Lars; Rasmussen, Lone Banke; Laurberg, Peter (2011). «Epidemiology of subtypes of hyperthyroidism in Denmark: a population-based study». European Journal of Endocrinology. 164 (5): 801–809. doi:10.1530/EJE-10-1155. ISSN 0804-4643. PMID 21357288.
- ^ Pokhrel, Binod; Bhusal, Kamal (2020), «Graves Disease», StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 28846288, retrieved 2020-12-04
- ^ Mathew Graves at Who Named It?
- ^ Graves, RJ. Newly observed affection of the thyroid gland in females Archived 2016-03-31 at the Wayback Machine. (Clinical lectures.) London Medical and Surgical Journal (Renshaw), 1835; 7 (part 2): 516–517. Reprinted in Medical Classics, 1940;5:33–36.
- ^ Von Basedow, KA. Exophthalmus durch Hypertrophie des Zellgewebes in der Augenhöhle. [Casper’s] Wochenschrift für die gesammte Heilkunde, Berlin, 1840, 6: 197–204; 220–228. Partial English translation in: Ralph Hermon Major (1884–1970): Classic Descriptions of Disease. Springfield, C. C. Thomas, 1932. 2nd edition, 1939; 3rd edition, 1945.
- ^ Von Basedow, KA. «Die Glotzaugen». [Casper’s] Wochenschrift für die gesammte Heilkunde, Berlin, 1848: 769–777.
- ^ a b c d e f g Basedow syndrome or disease at Who Named It? – the history and naming of the disease
- ^ a b c d Robinson, Victor, ed. (1939). «Exophthalmic goiter, Basedow disease, Grave disesase». The Modern Home Physician, A New Encyclopedia of Medical Knowledge. WM. H. Wise & Company (New York)., pages 82, 294, and 295.
- ^ Goiter, Diffuse Toxic at eMedicine
- ^ Flajani, G. Sopra un tumor freddo nell’anterior parte del collo broncocele. (Osservazione LXVII). In Collezione d’osservazioni e reflessioni di chirurgia. Rome, Michele A Ripa Presso Lino Contedini, 1802;3:270–273.
- ^ Testa, AG. Delle malattie del cuore, loro cagioni, specie, segni e cura. Bologna, 1810. 2nd edition in 3 volumes, Florence, 1823; Milano 1831; German translation, Halle, 1813.
- ^ Giuseppe Flajani at Who Named It?
- ^ Parry CH (1825). «Enlargement of the thyroid gland in connection with enlargement or palpitations of the heart». Collections from the unpublished medical writings of C. H. Parry. London. pp. 111–129.
According to Garrison, Parry first noted the condition in 1786. He briefly reported it in his Elements of Pathology and Therapeutics, 1815. Reprinted in Medical Classics, 1940, 5: 8–30
- ^ Hull G (June 1998). «Caleb Hillier Parry 1755-1822: a notable provincial physician». Journal of the Royal Society of Medicine. 91 (6): 335–8. doi:10.1177/014107689809100618. PMC 1296785. PMID 9771526.
- ^ Caleb Hillier Parry at Who Named It?
- ^ Sayyid Ismail Al-Jurjani. Thesaurus of the Shah of Khwarazm.
- ^ Ljunggren JG (August 1983). «[Who was the man behind the syndrome: Ismail al-Jurjani, Testa, Flagani, Parry, Graves or Basedow? Use the term hyperthyreosis instead]». Läkartidningen. 80 (32–33): 2902. PMID 6355710.
- ^ «水嶋ヒロ・絢香、2ショット会見で結婚報告 絢香はバセドウ病を告白、年内で休業へ» (in Japanese). Oricon. April 3, 2009. Archived from the original on December 8, 2015. Retrieved November 19, 2015.
- ^ «絢香、初のセルフ・プロデュース・アルバムが発売決定!» (in Japanese). CDJournal. December 1, 2011. Archived from the original on October 15, 2015. Retrieved November 19, 2015.
- ^ Shepley, Carol Ferring (2008). Movers and Shakers, Scalawags and Suffragettes: Tales from Bellefontaine Cemetery. St. Louis, Missouri: Missouri History Museum.
- ^ Altman LK (1991-05-28). «The Doctor’s World — A White House Puzzle: Immunity Ailments-Science Section». The New York Times. Archived from the original on 2013-05-08. Retrieved 2013-02-27.
- ^ Islam S (2017-01-23). «Thyroid gland – Hyperplasia / goiter – Graves disease». Pathologyoutlines.com. Archived from the original on 2016-12-14. Retrieved 2017-01-25.
- ^ Oldenburg A (2011-06-24). «Update: Missy Elliott ‘completely managing’ Graves’ disease». USA Today. Gannett.
- ^ «Famous People with Graves’ Disease». HRFnd. December 15, 2013. Retrieved 2018-02-22.
- ^ Kuhlenbeck M (June 29, 2016). «Marty Feldman versus the Suits». Jewish Currents. Retrieved 2018-02-22.
Viewers also could not help being amazed by his bulging eyes, which had resulted from a botched operation for Graves’ disease.
- ^ Rota G. «Facts About Sia Furler | Popsugar Celebrity Australia». Popsugar.com.au. Archived from the original on 2015-02-09. Retrieved 2016-09-10.
- ^ Guart, Al (March 31, 2002). «Rare Disease Could Whack Sammy Bull». New York Post. Archived from the original on January 11, 2012. Retrieved January 28, 2020.
- ^ «Hamilton talks about his disease on his podcast». YouTube. Archived from the original on 2017-09-08.
- ^ «Crossover Crooner: The Strange Comeback of Germany’s Wannabe Johnny Cash». Spiegel.de. 2013-02-07. Archived from the original on 2014-11-19. Retrieved 2014-07-27.
- ^ Spicer P (1998). Herbert Howells. Bridgend: Seren. p. 44. ISBN 1-85411-233-3.
- ^ «Yayoi Kusama by Grady T. Turner». Bomb Magazine. January 1, 1999. Retrieved May 29, 2020.
- ^ «Revolutionary First Lady: the life and struggles of Lenin’s wife». Russia Beyond. Archived from the original on 2018-04-18. Retrieved 2018-04-18.
- ^ «Barbara Leigh». Home.rmci.net. Archived from the original on 2012-07-10. Retrieved 2013-02-27.
- ^ «[歌手 増田恵子さん]バセドー病(1)マイク持つ手が震える». Yomiuri Shimbun. 2011-08-04. Retrieved 2020-02-01.
- ^ «[歌手 増田恵子さん]バセドー病(2)同じ病 姉の存在が支えに». Yomiuri Shimbun. 2011-08-11. Retrieved 2020-02-01.
- ^ «[歌手 増田恵子さん]バセドー病(3)ツアー中、甲状腺腫れ上がる». Yomiuri Shimbun. 2011-08-18. Retrieved 2020-02-01.
- ^ «[歌手 増田恵子さん]バセドー病(4)病気公表 無理せず我慢せず». Yomiuri Shimbun. 2011-08-25. Retrieved 2020-02-01.
- ^ «親子知新». www3.bigcosmic.com. Archived from the original on May 15, 2007. Retrieved December 18, 2017.
- ^ Rupert Murray «Meet the Climate Sceptics» Archived 2013-10-22 at the Wayback Machine, Storyville, 3 February 2011.
- ^ «Sophia Parnok, Russia’s Sappho».
- ^ Burgin, Diana Lewis (1992). «Sophia Parnok and the Writing of a Lesbian Poet’s Life». Slavic Review. 51 (2): 214–231. doi:10.2307/2499528. JSTOR 2499528. S2CID 163967264.
- ^ https://www.king.org/event/the-esoterics-parnok-in-that-infinite-moment/[permanent dead link]
- ^ Simon, Bernard (31 May 2013). «This memorial is poetic justice for Sir Cecil Spring Rice». telegraph.co.uk. Archived from the original on 2014-03-12. Retrieved 2014-08-25.
- ^ «Christina Rossetti». Poetry Foundation. Archived from the original on 2016-04-17. Retrieved 2016-09-10.
- ^ Wolf M (March 18, 1990). «There is Nothing Like This Dame». New York Times. Archived from the original on August 10, 2016. Retrieved 2015-10-19.
- ^ «Biography». Archived from the original on 2015-07-16. Retrieved 2015-07-16.
- ^ Melas C (February 21, 2018). «Wendy Williams announces show hiatus due to Graves’ disease». CNN. Retrieved February 21, 2018.
- ^ «Act Yasukawa Returns To Ring After Five Years Away». 15 November 2020.
- ^ Svevo, Italo (2003). Zeno’s conscience : a novel (1st Vintage International ed.). Vintage Books. pp. 315–321. ISBN 0375727760.
- ^ Scarponi, Mattia (19 August 2017). «Il morbo di Basedow: lo sfinimento tra Zeno e la realtà». theWise Magazine (in Italian). Retrieved 25 March 2020.
- ^ «Thyroid». Mayo Clinic. Archived from the original on 4 November 2016. Retrieved 1 November 2016.
External links[edit]
- «Graves’ disease». Genetics Home Reference. U.S. National Library of Medicine.
- https://www.ncbi.nlm.nih.gov/gene/?term=graves about graves on ncbi
Смотреть что такое БАЗЕДОВА БОЛЕЗНЬ в других словарях:
БАЗЕДОВА БОЛЕЗНЬ
этим именем называется заболевание, описанное впервые в 1840 г. Базедовым, имеющая следующие симптомы: сердцебиение, ускорение сердечной деятельности, … смотреть
БАЗЕДОВА БОЛЕЗНЬ
заболевание, связанное с повышением функции щитовидной железы, описанное немецким врачом К. Базедовом (К. Basedow, 1799—1854), то же, что Зоб д… смотреть
БАЗЕДОВА БОЛЕЗНЬ
БАЗЕДОВА БОЛЕЗНЬ, заболевание,
связанное с повышением функции щитовидной железы, описанное немецким врачом
К. Базедовом (К. Basedow, 1799- 1854), то … смотреть
БАЗЕДОВА БОЛЕЗНЬ
Базедова болезнь — этим именем называется заболевание, описанное впервые в 1840 г. Базедовым, имеющая следующие симптомы: сердцебиение, ускорение сердечной деятельности, усиленную пульсацию головных и шейных сосудов. Впоследствии к этим первоначальным симптомам заболевания присоединяется опухание щитовидной железы (зоб) и выпячивание глазных яблок (Ехоphtalmus). Причина заболевания лежит, по всем вероятиям, в паралитическом состоянии сосудодвигательных нервов головы и шеи, заложенных в шейном симпатическом сплетении. Болезнь эта встречается преимущественно у женщин, особенно в молодые годы, и имеет очень продолжительное течение (месяцы и даже годы). Часто базед. болезнь появляется внезапно, после ранения головы, сильного испуга или психического возбуждения. Лечение ограничивается, по преимуществу, укрепляющей диетой, железом, хинином, переменой климата, речными купаньями и применением гальванизации симпатического шейного сплетения.<br><br><br>… смотреть
БАЗЕДОВА БОЛЕЗНЬ
БАЗЕДОВА БОЛЕЗНЬпреимущественно у женщин, выражающаяся сердцебиением, выпучиванием глаз и сильным развитием зоба. Названа по имени д-ра Базедова, описа… смотреть
БАЗЕДОВА БОЛЕЗНЬ
1) Орфографическая запись слова: базедова болезнь2) Ударение в слове: баз`едова бол`езнь3) Деление слова на слоги (перенос слова): базедова болезнь4) Ф… смотреть
БАЗЕДОВА БОЛЕЗНЬ
(диффузный токсический зоб, болезнь Грейвса, болезнь Флаяни, болезнь Перри) — заболевание при котором имеется равномерное, диффузное увеличение щитовидной железы, избыточная продукция тиреиодных гормонов (гормонов щитовидной железы) и изменения в органах и тканях, вызванные большим количеством этих гормонов. Основные симптомы: увеличение щитовидной железы, утомляемость, общая слабость, рассеянность, повышенная раздражительность, сердцебиение, дрожание, потливость, похудание (при сохраненном или повышенном аппетите) и др. Лечение: терапевтическое (подавление функции щитовидной железы, в том числе радиоактивным йодом) и хирургическое (удаление части щитовидной железы).
Источник: «Медицинская Популярная Энциклопедия»… смотреть
БАЗЕДОВА БОЛЕЗНЬ
IБазе́дова боле́знь (morbus Basedowi; К. А. von Basedow, нем. врач, 1799—1854)см. Зоб диффузный токсический.
IIБазе́дова боле́знь (morbus Basedowi; K…. смотреть
БАЗЕДОВА БОЛЕЗНЬ
exophthalmic [toxic] goiter, (exophthalmic) hyperthyroidism, Graves’ [Basedow’s, Parry’s] disease, hyperthyroidism, thyrotoxicosis, thyroid cachexia
БАЗЕДОВА БОЛЕЗНЬ
1) basedowismo
2) gozzo basedowiano
3) gozzo esoftalmico
4) malattia di Basedow
5) malattia di Graves
6) morbo di Basedow
7) morbo di Flajani-Basedow… смотреть
БАЗЕДОВА БОЛЕЗНЬ
базедова болезнь (morbus Basedowi; К. A. von Basedow, 1799-1854, нем. врач) — см. Зоб диффузный токсический.
БАЗЕДОВА БОЛЕЗНЬ
Жарг. мол. Шутл. О состоянии удивления (когда у человека широко раскрыты глаза). Максимов, 21.
БАЗЕДОВА БОЛЕЗНЬ
1) goitre exophtalmique
2) goitre toxique
3) maladie de Basedow, maladie de Basedow-Graves
4) maladie de Graves
БАЗЕДОВА БОЛЕЗНЬ
Начальная форма — Базедова болезнь, винительный падеж, единственное число, женский род, неодушевленное
БАЗЕДОВА БОЛЕЗНЬ
(morbus Basedowi; К. A. von Basedow, 1799-1854, нем. врач) см. Зоб диффузный токсический.
Болезнь Грейвса: причины появления, симптомы, диагностика и способы лечения.
Определение
Болезнь Грейвса (другие названия – Базедова болезнь, токсический зоб) – наследственное аутоиммунное заболевание, которое может развиться в любом возрасте. Женщины болеют примерно в 5 раз чаще, чем мужчины. Болезнь характеризуется избыточной выработкой тиреоидных гормонов, что приводит к тиреотоксикозу (избытку гормонов щитовидной железы в кровеносном русле).
Замечено, что патологический процесс часто запускается в ситуациях, когда организм испытывает стресс.
Причины появления болезни Грейвса
Болезнь Грейвса связывают с врожденным дефектом специфических Т-лимфоцитов (в организме Т-лимфоциты регулируют скорость защитной реакции, ее продолжительность, обеспечивают клеточный иммунитет). Вследствие срыва иммунологической толерантности происходит активация В-лимфоцитов, продуцирующих тиреостимулирующие иммуноглобулины – АТ-рТТГ. АТ-рТТГ воздействуют на рецепторы к ТТГ и начинают стимулировать клетки щитовидной железы. Это приводит к выраженному повышению выработки тиреоидных гормонов (Т4 и Т3), а также к увеличению щитовидной железы в размерах (гипертрофии).
Спусковым механизмом нарушения иммунологической толерантности может стать вирусная инфекция, стресс, курение.
При болезни Грейвса щитовидная железа может увеличиться до такой степени, что будет видна визуально. При отсутствии лечения она начинает сдавливать пищевод и трахею, вызывая нарушения глотания и дыхания.
Классификация заболевания
Классифицируется болезнь Грейвса по размеру зоба, а также по степени тяжести синдрома тиреотоксикоза.
В России до настоящего времени обычно используется классификация эндемического зоба по О.В. Николаеву, предложенная еще в 1955 году.
0-я степень – щитовидная железа не видна и не пальпируется;
1-я степень – щитовидная железа не видна, но пальпируется и виден при глотании перешеек;
2-я степень – щитовидная железа видна во время глотания, пальпируется, форма шеи не изменена;
3- я степень – щитовидная железа видна, меняется контур шеи («толстая шея»);
4-я степень – большой зоб, нарушающий конфигурацию шеи;
5-я степень – огромный зоб, сдавливающий пищевод и трахею.
Классификация по тяжести тиреотоксикоза
Субклинический период – заболевание устанавливается на основании данных гормонального исследования, клиническая картина может отсутствовать или быть стертой.
Манифестный период – присутствует развернутая клиническая картина заболевания.
Осложненный период – присутствуют осложнения (мерцательная аритмия, сердечная недостаточность, тирогенная относительная надпочечниковая недостаточность, дистрофические изменения паренхиматозных органов (печени, селезенки и т.д.), психоз, значительный дефицит массы тела).
Симптомы болезни Грейвса
Болезнь Грейвса отличается большим разнообразием клинических проявлений. Больные предъявляют жалобы на учащенное сердцебиение (тахикардию), аритмию, жар (непереносимость теплых и душных помещений), сильное потоотделение, ощущение внутренней дрожи, потерю массы тела при хорошем аппетите, частый неоформленный стул. Характерен подъем систолического («верхнего») давления и снижение диастолического (нижнего). Многие страдают из-за нарушения сна, быстрой утомляемости и снижения работоспособности. Отмечаются отеки ног, слабость мышц, неприятное чувство сжатости в области шеи.
Второе название заболевания – Базедова болезнь – свидетельствует о том, что у пациентов нередко регистрируются офтальмологические нарушения (эндокринная офтальмопатия), которые проявляются пучеглазием (поражением мягких тканей глазницы). Можно услышать жалобы на регулярное слезотечение, давление и «песок» в глазах, двоение, отечность век, светобоязнь.
При пальпаторном обследовании примерно у 80% пациентов удается выявить увеличенную щитовидную железу.
У пациентов репродуктивного возраста может наблюдаться снижение либидо (у мужчин), для женщин характерны нарушения менструального цикла.
Как правило, симптомы развиваются быстро, поэтому пациенты приходят на прием к врачу в течение 6-12 мес. после их манифестации.
Диагностика болезни Грейвса
При подозрении на наличие у пациента тиреотоксикоза следует исследовать уровень ТТГ высокочувствительным методом.
При пониженном показателе уровня ТТГ пациенту проводится определение уровня св. Т4 и св. Т3: если хотя бы один из них повышен – речь идет о манифестном тиреотоксикозе, если оба показателя в норме – о субклиническом.
В число обязательных входят анализы на антитела к тиреоидной пероксидазе и к тиреоглобулину.
После подтверждения тиреотоксикоза проводится диагностика с целью выявления конкретного заболевания. Ультразвуковая картина болезни Грейвса не специфичная и характерна для других аутоиммунных заболеваний щитовидной железы. Кроме того, при болезни Грейвса могут определяться высокие уровни антитиреоидных антител – ат-ТПО и ат-ТГ. Однако вкупе с другими признаками заболевания и УЗИ, и анализ крови на ат-ТПО и ат-ТГ могут помочь в диагностике.
Большее диагностическое значение имеет определение уровня антител к рецепторам ТТГ (ат-рТТГ).
К каким врачам обращаться
В связи патологическими состояниями, сопутствующими болезни Грейвса, пациенты (особенно пожилые) нередко находятся под наблюдением кардиологов, терапевтов, гастроэнтерологов, а к
эндокринологам
попадают только при нарастании клинической картины основного заболевания. Поэтому всех пациентов с впервые выявленной необъяснимой сердечной недостаточностью, мерцательной аритмией, синдромом хронической усталости необходимо обследовать по поводу тиреотоксикоза.
Лечение болезни Грейвса
Консервативная терапия
Консервативное лечение препаратами из группы тионамидов (препаратами, блокирующими выработку гормонов щитовидной железы) показано пациентам с умеренно увеличенным объемом щитовидной железы (до 40 мл) и без серьезных тиреотоксических осложнений. Если через 1-2 года после консервативной терапии произошел рецидив, назначать ее повторно не имеет смысла.
Радиотерапия
Терапия радиоактивным йодом выполняется с целью разрушения щитовидной железы. Метод неинвазивен, достаточно эффективен и лишен побочных эффектов, которые могут развиться после хирургической операции.
Оперативное лечение
Назначают пациентам в случае, если щитовидная железа имеет очень большие размеры, узлы или есть подозрение на их озлокачествление. Удаление щитовидной железы позволяет добиться послеоперационного гипотиреоза, который компенсируется за счет приема левотироксина и не приводит к снижению качества жизни пациентов.
Лечение эндокринной офтальмопатии
Рекомендуются глазные капли с дексаметазоном и ношение солнцезащитных очков.
Начиная со 2-й степени офтальмопатии пациентам назначают глюкокортикоиды. При резистентности к лечению или при рецидивах назначают рентгенотерапию на область орбиты. В сложных случаях прибегают к хирургической декомпрессии глазницы с удалением ретробульбарной клетчатки, а при необходимости — костных стенок глазницы. Косметические операции проводят, если требуется коррекция экзофтальма и косоглазия, развивающихся в результате фиброзных процессов.
Осложнения
Тиреотоксикоз негативно воздействует не только на щитовидную железу, но и на другие органы и системы. Чтобы избежать инвалидизации, требуется своевременное и правильное лечение.
Наиболее опасное осложнение болезни Грейвса — тиреотоксический криз. Он проявляется резким возбуждением, повышением температуры до 40 оС, сердцебиением до 200 ударов в минуту, усилением диспепсии (тошноты, рвоты, жажды). Затем могут появиться признаки надпочечниковой недостаточности (нарушение микроциркуляции, нитевидный пульс). Эта ситуация требует принятия срочных мер для снижения уровня тиреоглобулина и проявлений криза. Факторами развития тиреотоксического криза могут быть стресс, различные заболевания, избыточная физическая нагрузка, и т.д.
К осложнениям диффузного токсического зоба относятся осложнения после его хирургического лечения: гипопаратиреоз, повреждение возвратного гортанного нерва, в результате чего развивается осиплость голоса, кровотечения, аллергические реакции на препараты.
Профилактика болезни Грейвса
Прогноз при своевременной диагностике и надлежащем лечении благоприятный. Благодаря доступной заместительной терапии удается добиться нормализации метаболических процессов и исчезновения клинических проявлений заболевания.
Профилактикой диффузного токсического зоба является отказ от курения, приема лекарств, в побочных эффектах которых упоминается влияние на выработку тиреоидных гормонов, а также йод-содержащих препаратов, соблюдение оптимального режима дня для минимизации стрессовых ситуаций и регулярный контроль ТТГ.
Источники:
- Федеральные клинические рекомендации по диагностике и лечению тиреотоксикоза с диффузным зобом (диффузный токсический зоб, болезнь Грейвса-Базедова), узловым/многоузловым зобом. Трошина Е.А., Свириденко Н.Ю., Ванушко В.Э и соавт. М. 2014.
- Клинические рекомендации «Диффузный токсический зоб». Дети. Разраб. Российская ассоциация эндокринологов. – 2021. – 35 с.
- Фадеев В.В. Диагностика и лечение болезни Грейвса. Медицинский совет, журнал. №4/2014. С. 44-48.
- Ванушко. В.Э., Фадеев В.В. Болезнь Грейвса (клиническая лекция). Эндокринная хирургия, журнал. №1/2013. С. 23-33.
ВАЖНО!
Информацию из данного раздела нельзя использовать для самодиагностики и самолечения. В случае боли или иного обострения заболевания диагностические исследования должен назначать только лечащий врач. Для постановки диагноза и правильного назначения лечения следует обращаться к Вашему лечащему врачу.
Для корректной оценки результатов ваших анализов в динамике предпочтительно делать исследования в одной и той же лаборатории, так как в разных лабораториях для выполнения одноименных анализов могут применяться разные методы исследования и единицы измерения.
Информация проверена экспертом

Лишова Екатерина Александровна
Высшее медицинское образование, опыт работы — 19 лет
Поделитесь этой статьей сейчас
Рекомендации
-
13060
01 Марта
-
13059
23 Февраля
-
4419
21 Февраля
Похожие статьи
Тиреоидит
Тиреоидит: причины появления, симптомы, диагностика и способы лечения.
Подострый тиреоидит
Подострый тиреоидит: причины появления, симптомы, диагностика и способы лечения.
Микседема
Микседема: причины появления, симптомы, диагностика и способы лечения.
##Два вскрытия Безедова За этим термином стоят два вскрытия, произведённые доктором Карлом фон Базедовым. Первое вскрытие он сделал в 1838 году, изучая тело своей шестимесячной дочери. Девочка болела всего 8 дней. Началось с насморка, затем раздулись лимфоузлы на шее. Они стали размером с каштан и загноились. Голова разбухла, а вилочковая железа увеличилась так, что давила на трахею и душила девочку, пока та не умерла. Вскрытие показало, что лёгкие, печень, селезёнка и кишечник усыпаны бугорками размером с просяное зерно. То был недавно описанный в литературе милиарный туберкулёз. 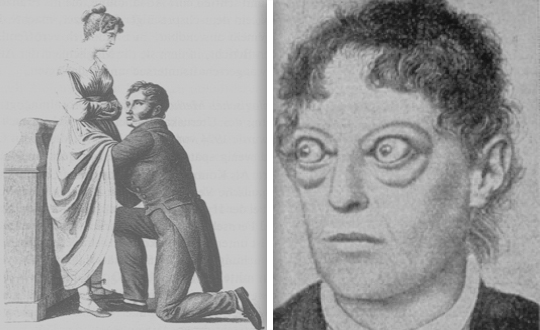 Слева: дозволенный приличиями способ, которым в первой половине XIX века европейские врачи проводили осмотр таза и живота пациенток; справа — изображение мадам G., первой пациентки с диффузным токсическим зобом, которую лечил Карл фон Базедов. Базедов имел собственную практику в саксонском городке Мерзебург. Он лечил взрослых и детей, причём детей бедняков — бесплатно. Незадолго до болезни дочери у него был подобный случай: несмотря на энергичные меры, ребёнок умер с теми же симптомами. Теперь Базедов переживал, что принёс эту заразу к себе в дом. На город с 8 тысячами населения за многие годы всего четыре случая детского туберкулёза, из них один — в семье доктора. Что ж, это его профессиональный риск. И в память о дочери он решил сделать нечто особенное в своей работе. А именно, собрать интересные случаи из мерзебургской практики в статье, достойной всеобщего внимания, и в заключение рассказать о своей девочке. ##Мадам G Подбирая близкие по теме случаи, Базедов остановился на золотухе, как называли в то время проблемы с железами. Пациентку «мадам G.» автор наблюдал 14 лет: он помнил её ещё красивой, тоненькой 19-летней девушкой, у которой распухли узлы на шее. Два года спустя она перенесла четырёхдневную лихорадку, оставившую по себе набухание печени, которое, по мнению Базедова, вызвало снижение частоты пульса. С этим симптомом доктор боролся общими и местными кровопусканиями, энергичным втиранием в кожу ртути и хинином. Наконец при помощи соды с белладонной увеличение печени сумели устранить и добиться выздоровления. Но через год возникла новая напасть: острый ревматизм, путешествующий о всему телу из одного сустава в другой. С заболеванием суставов Базедов боролся средством, предписанным ещё Авиценной — настойкой семян безвременника с сулемой каждые три часа. Ревматизм прошёл, оставив по себе снижение массы тела, аменорею, сердцебиение, учащённое дыхание и симптом, который доктора тогда называли «тревога в груди». Эта самая тревога сменилась экзофтальмом: глаза больной выпучились так, что она не могла сомкнуть веки и спала с открытыми глазами. Вид её внушал людям страх. Но сама пациентка не ощущала никакого беспокойства. Она всегда отличалась бойким поведением, а теперь и вовсе резвилась как ребёнок. Весёлая и беспечная, она ходила в прохладную погоду нараспашку, и по городу пошли разговоры, что бедняжка тронулась умом и пора сдать её в королевский сумасшедший дом.  Карл Адольф фон Базедов (1799-1854) — немецкий врач, именем которого названа описанная им болезнь (диффузный токсический зоб). С единственного прижизненного портрета работы его племянника Франца Крюгера. Оригинал находится в мерзебургской больнице, где работал Базедов, ныне носящей его имя (Carl-von-Basedow-Klinikum). ##Мадам F Базедов стал изучать литературу по глазным болезням, вызывающим пучеглазие, но ни один диагноз для мадам G. не подходил: не было ухудшения зрения. Вылечить её удалось, когда у больной развился зоб. Уже был широко известен опыт Жан-Франсуа Куанде, прославленного на всю Европу. В одном высокогорном месте Швейцарии население сплошь ходило с зобами, пока Куанде не установил, что причиной всему нехватка иода. Иодом и наперстянкой Базедов сумел справиться с зобом и тахикардией. Больная теперь могла закрыть глаза. Вскоре она вышла замуж и родила, после чего от болезни осталась только бледность кожи и глаза слегка навыкате. Другая пациентка — мадам F., эффектная брюнетка, также жаловалась на сердцебиение, пучеглазие и зоб. Бойкостью она превосходила мадам G., и была так заметна, что мерзебургские граждане всё-таки отвезли её в королевский сумасшедший дом. Но Базедов ручался перед тамошними психиатрами, что она всегда владела собой, и вскоре мадам F. выписали. Муж поехал с ней на воды, к йодному источнику, где она слегка поправилась, а во время беременности и вовсе выздоровела. Симптомы вернулись после родов, но ушли с новой беременностью. ##Средство от пучеглазого зоба Таким образом, было обнаружено надёжное средство против «пучеглазого зоба» — беременность. Однако выяснилось, что этой болезнью страдают и мужчины. Герр М. стал для Базедова настоящей болью. Он со всеми теми же симптомами проявлял сходную беспечность, хотя достиг 45 лет и был уважаемым в городе «материалистом», как немцы называли бакалейщиков. Материалист часто ездил за товарами через границу и сам правил лошадьми, хотя Базедов предписывал ему беречь выпученные глаза от инфекции. В конце концов озорной герр М. потерял зрение и доктор ничем не смог ему помочь. На момент написания статьи больной был ещё жив, но крайне истощен, с трудом выдерживал приступы сердцебиения, а распухшая щитовидная железа мешала ему дышать. Обобщив симптомы 4 случаев диффузного токсического зоба, Базедов пришёл к выводу, что это особое заболевание, проявлящееся тахикардией, зобом и пучеглазием. По его мнению, возникало оно от дискразии («неправильно смешения») крови где-то в районе щитовидной железы и влечёт отравление всего организма. Никто в те времена не мог описать причины болезни точнее. О гормонах тогда и речи не было, и лишь спустя 120 лет выяснилось, что за аутоиммунным процессом стоят определённые антитела в крови. ##Случай на похоронах В конце статьи Базедов привёл случай своей несчастной дочери. Это было своеобразное посвящение, так как статья не касалась туберкулёза. Чтобы сделать автору приятно, редакторы берлинского врачебного еженедельника поставили статью в номер, который вышел в день рождения Базедова, 28 марта 1840 года. Саксонский король был очень польщён, что в его маленьком государстве описали новую болезнь, и сделал Базедова «медицинским советником». Когда 8 лет спустя решался вопрос, кому из мерзебургских врачей стать окружным доктором и главным врачом городской больницы, из восьми кандидатов был отобран именно Базедов. Это назначение его и погубило. Окружной врач должен был вскрывать все тела умерших по неизвестным причинам. В апреле 1854-го Базедов заразился, делая вскрытие погибшего от неизученной трехдневной лихорадки. Что это было, до сих пор не ясно, но должно быть, нечто весьма опасное, потому что умерли богаделка, одевавшая тело доктора, и кучер, который правил катафалком на похоронах. После такой героической гибели коллеги решили увековечить имя мерзебургского врача Базедова. И хотя не он первым описал диффузный токсический зоб, триаду его главных симптомов — пучеглазие, тахикардию, зоб — назвали мерзебургской триадой, а само заболевание — базедовой болезнью.
Михаил Шифрин
Источники
- Surasi DSS., Wang X., Bathala TK., Hwang H., Arora S., Westphalen AC., Chang SD., Turkbey B. The impact and collateral damage of COVID-19 on prostate MRI and guided biopsy operations: Society of Abdominal Radiology Prostate Cancer Disease-Focused Panel survey analysis. // Abdom Radiol (NY) — 2021 — Vol — NNULL — p.; PMID:33904992
- Mehdorn M., Lübbert C., Chaberny IF., Gockel I., Jansen-Winkeln B. Mechanical plus oral bowel preparation with paromomycine and metronidazole reduces infectious complications in elective colorectal surgery: a matched case-control study. // Int J Colorectal Dis — 2021 — Vol — NNULL — p.; PMID:33895874
- Möllers T., Schwab M., Gildein L., Hoffmeister M., Albert J., Brenner H., Jäger S. Second-generation colon capsule endoscopy for detection of colorectal polyps: Systematic review and meta-analysis of clinical trials. // Endosc Int Open — 2021 — Vol9 — N4 — p.E562-E571; PMID:33860073
- Lee J., Wallace MB. State of the Art: The Impact of Artificial Intelligence in Endoscopy 2020. // Curr Gastroenterol Rep — 2021 — Vol23 — N5 — p.7; PMID:33855659
- Cheng Y., Zhong C., Wu W., Xi X., Luo M., Chen Y., Zhang B. Association between anxiety, depression, and bowel air bubbles at colonoscopy: a prospective observational study. // Ann Palliat Med — 2021 — Vol10 — N3 — p.3247-3257; PMID:33849109
- Dong W., Liu D., Zhang T., You Q., Huang F., Wu J. Oral delivery of staphylococcal nuclease ameliorates DSS induced ulcerative colitis in mice via degrading intestinal neutrophil extracellular traps. // Ecotoxicol Environ Saf — 2021 — Vol215 — NNULL — p.112161; PMID:33812202
- Sey M., Yan B., McDonald C., Segal D., Friedland J., Puka K., Jairath V. A randomized controlled trial of high volume simethicone to improve visualization during capsule endoscopy. // PLoS One — 2021 — Vol16 — N4 — p.e0249490; PMID:33793636
- El Bizri M., El Sheikh M., Lee GE., Sewitch MJ. Mobile health technologies supporting colonoscopy preparation: A systematic review and meta-analysis of randomized controlled trials. // PLoS One — 2021 — Vol16 — N3 — p.e0248679; PMID:33735320
- Zhang Y., Liu R., Wang J., Yan S., Guo Z. To assess the effective and safety of compound glutamine entersoluble capsules in irritable bowel syndrome: A protocol for systematic review and meta-analysis. // Medicine (Baltimore) — 2021 — Vol100 — N10 — p.e25098; PMID:33725903
- Fallahi P., Ferrari SM., Elia G., Ragusa F., Paparo SR., Antonelli A. L-T4 Therapy in Enteric Malabsorptive Disorders. // Front Endocrinol (Lausanne) — 2021 — Vol12 — NNULL — p.626371; PMID:33708175