A sample of silicon dioxide |
|
| Names | |
|---|---|
| IUPAC name
Silicon dioxide |
|
Other names
|
|
| Identifiers | |
|
CAS Number |
|
| ChEBI |
|
| ChemSpider |
|
| ECHA InfoCard | 100.028.678 |
| EC Number |
|
| E number | E551 (acidity regulators, …) |
|
Gmelin Reference |
200274 |
| KEGG |
|
| MeSH | Silicon+dioxide |
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
|
CompTox Dashboard (EPA) |
|
|
InChI
|
|
| Properties | |
|
Chemical formula |
SiO2 |
| Molar mass | 60.08 g/mol |
| Appearance | Transparent solid (Amorphous) White/Whitish Yellow (Powder/Sand) |
| Density | 2.648 (α-quartz), 2.196 (amorphous) g·cm−3[1] |
| Melting point | 1,713 °C (3,115 °F; 1,986 K) (amorphous)[1]: 4.88 to |
| Boiling point | 2,950 °C (5,340 °F; 3,220 K)[1] |
|
Magnetic susceptibility (χ) |
−29.6·10−6 cm3/mol |
| Thermal conductivity | 12 (|| c-axis), 6.8 (⊥ c-axis), 1.4 (am.) W/(m⋅K)[1]: 12.213 |
|
Refractive index (nD) |
1.544 (o), 1.553 (e)[1]: 4.143 |
| Hazards | |
| NFPA 704 (fire diamond) |
0 0 0 |
| NIOSH (US health exposure limits): | |
|
PEL (Permissible) |
TWA 20 mppcf (80 mg/m3/%SiO2) (amorphous)[2] |
|
REL (Recommended) |
TWA 6 mg/m3 (amorphous)[2] Ca TWA 0.05 mg/m3[3] |
|
IDLH (Immediate danger) |
3000 mg/m3 (amorphous)[2] Ca [25 mg/m3 (cristobalite, tridymite); 50 mg/m3 (quartz)][3] |
| Related compounds | |
|
Related diones |
Carbon dioxide
Germanium dioxide |
|
Related compounds |
Silicon monoxide
Silicon sulfide |
| Thermochemistry | |
|
Std molar |
42 J·mol−1·K−1[4] |
|
Std enthalpy of |
−911 kJ·mol−1[4] |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, most commonly found in nature as quartz.[5][6] In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an electrical insulator), and as components in the food and pharmaceutical industries.
Structure[edit]
Structural motif found in α-quartz, but also found in almost all forms of silicon dioxide
Typical subunit for low pressure silicon dioxide
Relationship between refractive index and density for some SiO2 forms[7]
In the majority of silicon dioxides, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atom (see 3-D Unit Cell). Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear molecule. The starkly different structures of the dioxides of carbon and silicon are a manifestation of the double bond rule.
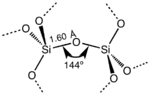
SiO2 has several distinct crystalline forms, but they almost always have the same local structure around Si and O. In α-quartz the Si–O bond length is 161 pm, whereas in α-tridymite it is in the range 154–171 pm. The Si–O–Si angle also varies between a low value of 140° in α-tridymite, up to 180° in β-tridymite. In α-quartz, the Si–O–Si angle is 144°.[8]
Polymorphism[edit]
Alpha quartz is the most stable form of solid SiO2 at room temperature. The high-temperature minerals, cristobalite and tridymite, have both lower densities and indices of refraction than quartz. The transformation from α-quartz to beta-quartz takes place abruptly at 573 °C. Since the transformation is accompanied by a significant change in volume, it can easily induce fracturing of ceramics or rocks passing through this temperature limit.[9] The high-pressure minerals, seifertite, stishovite, and coesite, though, have higher densities and indices of refraction than quartz.[10] Stishovite has a rutile-like structure where silicon is 6-coordinate. The density of stishovite is 4.287 g/cm3, which compares to α-quartz, the densest of the low-pressure forms, which has a density of 2.648 g/cm3.[11] The difference in density can be ascribed to the increase in coordination as the six shortest Si–O bond lengths in stishovite (four Si–O bond lengths of 176 pm and two others of 181 pm) are greater than the Si–O bond length (161 pm) in α-quartz.[12]
The change in the coordination increases the ionicity of the Si–O bond.[13] More importantly, any deviations from these standard parameters constitute microstructural differences or variations, which represent an approach to an amorphous, vitreous, or glassy solid.
Faujasite silica, another polymorph, is obtained by dealumination of a low-sodium, ultra-stable Y zeolite with combined acid and thermal treatment. The resulting product contains over 99% silica, and has high crystallinity and specific surface area (over 800 m2/g). Faujasite-silica has very high thermal and acid stability. For example, it maintains a high degree of long-range molecular order or crystallinity even after boiling in concentrated hydrochloric acid.[14]
Molten SiO2[edit]
Molten silica exhibits several peculiar physical characteristics that are similar to those observed in liquid water: negative temperature expansion, density maximum at temperatures ~5000 °C, and a heat capacity minimum.[15] Its density decreases from 2.08 g/cm3 at 1950 °C to 2.03 g/cm3 at 2200 °C.[16]
Molecular SiO2[edit]
The molecular SiO2 has a linear structure like CO2. It has been produced by combining silicon monoxide (SiO) with oxygen in an argon matrix.
The dimeric silicon dioxide, (SiO2)2 has been obtained by reacting O2 with matrix isolated dimeric silicon monoxide, (Si2O2). In dimeric silicon dioxide there are two oxygen atoms bridging between the silicon atoms with an Si–O–Si angle of 94° and bond length of 164.6 pm and the terminal Si–O bond length is 150.2 pm. The Si–O bond length is 148.3 pm, which compares with the length of 161 pm in α-quartz. The bond energy is estimated at 621.7 kJ/mol.[17]
Natural occurrence[edit]
Geology[edit]
SiO2 is most commonly found in nature as quartz, which comprises more than 10% by mass of the Earth’s crust.[18] Quartz is the only polymorph of silica stable at the Earth’s surface. Metastable occurrences of the high-pressure forms coesite and stishovite have been found around impact structures and associated with eclogites formed during ultra-high-pressure metamorphism. The high-temperature forms of tridymite and cristobalite are known from silica-rich volcanic rocks. In many parts of the world, silica is the major constituent of sand.[19]
Biology[edit]
Even though it is poorly soluble, silica occurs in many plants such as rice. Plant materials with high silica phytolith content appear to be of importance to grazing animals, from chewing insects to ungulates. Silica accelerates tooth wear, and high levels of silica in plants frequently eaten by herbivores may have developed as a defense mechanism against predation.[20][21]
Silica is also the primary component of rice husk ash, which is used, for example, in filtration and as supplementary cementitious material (SCM) in cement and concrete manufacturing.[citation needed]
For well over a 1000 million years, silicification in and by cells has been common in the biological world. In the modern world, it occurs in bacteria, single-celled organisms, plants, and animals (invertebrates and vertebrates).
Prominent examples include:
- Tests or frustules (i.e. shells) of diatoms, Radiolaria, and testate amoebae.[6]
- Silica phytoliths in the cells of many plants, including Equisetaceae, practically all grasses, and a wide range of dicotyledons.
- The spicules forming the skeleton of many sponges.
Crystalline minerals formed in the physiological environment often show exceptional physical properties (e.g., strength, hardness, fracture toughness) and tend to form hierarchical structures that exhibit microstructural order over a range of scales. The minerals are crystallized from an environment that is undersaturated concerning silicon, and under conditions of neutral pH and low temperature (0–40 °C).
Uses[edit]
Structural use[edit]
About 95% of the commercial use of silicon dioxide (sand) occurs in the construction industry, e.g. for the production of concrete (Portland cement concrete).[18]
Certain deposits of silica sand, with desirable particle size and shape and desirable clay and other mineral content, were important for sand casting of metallic products.[22] The high melting point of silica enables it to be used in such applications such as iron casting; modern sand casting sometimes uses other minerals for other reasons.
Crystalline silica is used in hydraulic fracturing of formations which contain tight oil and shale gas.[23]
Precursor to glass and silicon[edit]
Silica is the primary ingredient in the production of most glass. As other minerals are melted with silica, the principle of freezing point depression lowers the melting point of the mixture and increases fluidity. The glass transition temperature of pure SiO2 is about 1475 K.[24] When molten silicon dioxide SiO2 is rapidly cooled, it does not crystallize, but solidifies as a glass. Because of this, most ceramic glazes have silica as the main ingredient.
The structural geometry of silicon and oxygen in glass is similar to that in quartz and most other crystalline forms of silicon and oxygen with silicon surrounded by regular tetrahedra of oxygen centres. The difference between the glass and crystalline forms arises from the connectivity of the tetrahedral units: Although there is no long-range periodicity in the glassy network ordering remains at length scales well beyond the SiO bond length. One example of this ordering is the preference to form rings of 6-tetrahedra.[25]
The majority of optical fibers for telecommunication are also made from silica. It is a primary raw material for many ceramics such as earthenware, stoneware, and porcelain.
Silicon dioxide is used to produce elemental silicon. The process involves carbothermic reduction in an electric arc furnace:[26]
Fumed silica[edit]
Fumed silica, also known as pyrogenic silica, is prepared by burning SiCl4 in an oxygen-rich hydrogen flame to produce a «smoke» of SiO2.[11]
It can also be produced by vaporizing quartz sand in a 3000 °C electric arc. Both processes result in microscopic droplets of amorphous silica fused into branched, chainlike, three-dimensional secondary particles which then agglomerate into tertiary particles, a white powder with extremely low bulk density (0.03-.15 g/cm3) and thus high surface area.[27] The particles act as a thixotropic thickening agent, or as an anti-caking agent, and can be treated to make them hydrophilic or hydrophobic for either water or organic liquid applications
Manufactured fumed silica with maximum surface area of 380 m2/g
Silica fume is an ultrafine powder collected as a by-product of the silicon and ferrosilicon alloy production. It consists of amorphous (non-crystalline) spherical particles with an average particle diameter of 150 nm, without the branching of the pyrogenic product. The main use is as pozzolanic material for high performance concrete. Fumed silica nanoparticles can be successfully used as an anti-aging agent in asphalt binders.[28]
Food, cosmetic, and pharmaceutical applications[edit]
Silica, either colloidal, precipitated, or pyrogenic fumed, is a common additive in food production. It is used primarily as a flow or anti-caking agent in powdered foods such as spices and non-dairy coffee creamer, or powders to be formed into pharmaceutical tablets.[27] It can adsorb water in hygroscopic applications. Colloidal silica is used as a fining agent for wine, beer, and juice, with the E number reference E551.[18]
In cosmetics, silica is useful for its light-diffusing properties[29] and natural absorbency.[30]
Diatomaceous earth, a mined product, has been used in food and cosmetics for centuries. It consists of the silica shells of microscopic diatoms; in a less processed form it was sold as «tooth powder».[citation needed] Manufactured or mined hydrated silica is used as the hard abrasive in toothpaste.
Semiconductors[edit]
Silicon dioxide is widely used in the semiconductor technology
- for the primary passivation (directly on the semiconductor surface),
- as an original gate dielectric in MOS technology. Today when scaling (dimension of the gate length of the MOS transistor) has progressed below 10 nm silicon dioxide has been replaced by other dielectric materials like hafnium oxide or similar with higher dielectric constant compared to silicon dioxide,
- as a dielectric layer between metal (wiring) layers (sometimes up to 8-10) connecting elements and
- as a second passivation layer (for protecting semiconductor elements and the metallization layers) typically today layered with some other dielectrics like silicon nitride.
Because silicon dioxide is a native oxide of silicon it is more widely used compared to other semiconductors like gallium arsenide or indium phosphide.
Silicon dioxide could be grown on a silicon semiconductor surface.[31] Silicon oxide layers could protect silicon surfaces during diffusion processes, and could be used for diffusion masking.[32][33]
Surface passivation is the process by which a semiconductor surface is rendered inert, and does not change semiconductor properties as a result of interaction with air or other materials in contact with the surface or edge of the crystal.[34][35] The formation of a thermally grown silicon dioxide layer greatly reduces the concentration of electronic states at the silicon surface.[35] SiO2 films preserve the electrical characteristics of p–n junctions and prevent these electrical characteristics from deteriorating by the gaseous ambient environment.[33] Silicon oxide layers could be used to electrically stabilize silicon surfaces.[32] The surface passivation process is an important method of semiconductor device fabrication that involves coating a silicon wafer with an insulating layer of silicon oxide so that electricity could reliably penetrate to the conducting silicon below. Growing a layer of silicon dioxide on top of a silicon wafer enables it to overcome the surface states that otherwise prevent electricity from reaching the semiconducting layer.[34][36]
The process of silicon surface passivation by thermal oxidation (silicon dioxide) is critical to the semiconductor industry. It is commonly used to manufacture metal–oxide–semiconductor field-effect transistors (MOSFETs) and silicon integrated circuit chips (with the planar process).[34][36]
Other[edit]
Hydrophobic silica is used as a defoamer component.
In its capacity as a refractory, it is useful in fiber form as a high-temperature thermal protection fabric.[citation needed]
Silica is used in the extraction of DNA and RNA due to its ability to bind to the nucleic acids under the presence of chaotropes.[37]
Silica aerogel was used in the Stardust spacecraft to collect extraterrestrial particles.[38]
Pure silica (silicon dioxide), when cooled as fused quartz into a glass with no true melting point, can be used as a glass fibre for fibreglass.
Insecticide[edit]
Silicon dioxide has been researched for agricultural applications as a potential insecticide.[39][40]
Production[edit]
Silicon dioxide is mostly obtained by mining, including sand mining and purification of quartz.
Quartz is suitable for many purposes, while chemical processing is required to make a purer or otherwise more suitable (e.g. more reactive or fine-grained) product.[citation needed]
Precipitated silica[edit]
Precipitated silica or amorphous silica is produced by the acidification of solutions of sodium silicate. The gelatinous precipitate or silica gel, is first washed and then dehydrated to produce colorless microporous silica.[11] The idealized equation involving a trisilicate and sulfuric acid is:
Approximately one billion kilograms/year (1999) of silica were produced in this manner, mainly for use for polymer composites – tires and shoe soles.[18]
On microchips[edit]
Thin films of silica grow spontaneously on silicon wafers via thermal oxidation, producing a very shallow layer of about 1 nm or 10 Å of so-called native oxide.[41]
Higher temperatures and alternative environments are used to grow well-controlled layers of silicon dioxide on silicon, for example at temperatures between 600 and 1200 °C, using so-called dry oxidation with O2
or wet oxidation with H2O.[42][43]
The native oxide layer is beneficial in microelectronics, where it acts as electric insulator with high chemical stability. It can protect the silicon, store charge, block current, and even act as a controlled pathway to limit current flow.[44]
Laboratory or special methods[edit]
From organosilicon compounds[edit]
Many routes to silicon dioxide start with an organosilicon compound, e.g., HMDSO,[45] TEOS. Synthesis of silica is illustrated below using tetraethyl orthosilicate (TEOS).[46] Simply heating TEOS at 680–730 °C results in the oxide:
Similarly TEOS combusts around 400 °C:
TEOS undergoes hydrolysis via the so-called sol-gel process. The course of the reaction and nature of the product are affected by catalysts, but the idealized equation is:[47]
Other methods[edit]
Being highly stable, silicon dioxide arises from many methods. Conceptually simple, but of little practical value, combustion of silane gives silicon dioxide. This reaction is analogous to the combustion of methane:
However the chemical vapor deposition of silicon dioxide onto crystal surface from silane had been used using nitrogen as a carrier gas at 200–500 °C.[48]
Chemical reactions[edit]
Silicon dioxide is a relatively inert material (hence its widespread occurrence as a mineral. Silica is often used as inert containers for chemical reactions. At high temperatures, it is converted to silicon by reduction with carbon.
Fluorine reacts with silicon dioxide to form SiF4 and O2 whereas the other halogen gases (Cl2, Br2, I2) are unreactive.[11]
Most forms of silicon dioxide are attacked («etched») by hydrofluoric acid (HF) to produce hexafluorosilicic acid:[8]
- SiO2 + 6 HF → H2SiF6 + 2 H2O
Stishovite, which does not react to any significant degree[49])
HF is used to remove or pattern silicon dioxide in the semiconductor industry.
Silicon dioxide acts as a Lux–Flood acid, being able to react with bases under certain conditions. As it does not contain any hydrogen, non-hydrated silica cannot directly act as a Brønsted–Lowry acid. While silicon dioxide is only poorly soluble in water at low or neutral pH (typically, 2 × 10−4 M for quartz up to 10−3 M for cryptocrystalline chalcedony), strong bases react with glass and easily dissolve it. Therefore, strong bases have to be stored in plastic bottles to avoid jamming the bottle cap, to preserve the integrity of the recipient, and to avoid undesirable contamination by silicate anions.[50]
Silicon dioxide dissolves in hot concentrated alkali or fused hydroxide, as described in this idealized equation:[11]
Silicon dioxide will neutralise basic metal oxides (e.g. sodium oxide, potassium oxide, lead(II) oxide, zinc oxide, or mixtures of oxides, forming silicates and glasses as the Si-O-Si bonds in silica are broken successively).[8] As an example the reaction of sodium oxide and SiO2 can produce sodium orthosilicate, sodium silicate, and glasses, dependent on the proportions of reactants:[11]
.
Examples of such glasses have commercial significance, e.g. soda-lime glass, borosilicate glass, lead glass. In these glasses, silica is termed the network former or lattice former.[8] The reaction is also used in blast furnaces to remove sand impurities in the ore by neutralisation with calcium oxide, forming calcium silicate slag.
Silicon dioxide reacts in heated reflux under dinitrogen with ethylene glycol and an alkali metal base to produce highly reactive, pentacoordinate silicates which provide access to a wide variety of new silicon compounds.[51] The silicates are essentially insoluble in all polar solvent except methanol.
Silicon dioxide reacts with elemental silicon at high temperatures to produce SiO:[8]
Water solubility[edit]
The solubility of silicon dioxide in water strongly depends on its crystalline form and is three-four times higher for silica[clarification needed] than quartz; as a function of temperature, it peaks around 340 °C (644 °F).[52] This property is used to grow single crystals of quartz in a hydrothermal process where natural quartz is dissolved in superheated water in a pressure vessel that is cooler at the top. Crystals of 0.5–1 kg can be grown for 1–2 months.[8] These crystals are a source of very pure quartz for use in electronic applications.[11] Above the critical temperature of water 647.096 K (373.946 °C; 705.103 °F) and a pressure of 22.064 megapascals (3,200.1 psi) or higher, water is a supercritical fluid and solubility is once again higher than at lower temperatures.[53]
Health effects[edit]
Quartz sand (silica) as main raw material for commercial glass production
Silica ingested orally is essentially nontoxic, with an LD50 of 5000 mg/kg (5 g/kg).[18] A 2008 study following subjects for 15 years found that higher levels of silica in water appeared to decrease the risk of dementia. An increase of 10 mg/day of silica in drinking water was associated with a decreased risk of dementia of 11%.[54]
Inhaling finely divided crystalline silica dust can lead to silicosis, bronchitis, or lung cancer, as the dust becomes lodged in the lungs and continuously irritates the tissue, reducing lung capacities.[55] When fine silica particles are inhaled in large enough quantities (such as through occupational exposure), it increases the risk of systemic autoimmune diseases such as lupus[56] and rheumatoid arthritis compared to expected rates in the general population.[40]
Occupational hazard[edit]
Silica is an occupational hazard for people who do sandblasting or work with products that contain powdered crystalline silica. Amorphous silica, such as fumed silica, may cause irreversible lung damage in some cases but is not associated with the development of silicosis. Children, asthmatics of any age, those with allergies, and the elderly (all of whom have reduced lung capacity) can be affected in less time.[57]
Crystalline silica is an occupational hazard for those working with stone countertops, because the process of cutting and installing the countertops creates large amounts of airborne silica.[58] Crystalline silica used in hydraulic fracturing presents a health hazard to workers.[23]
Pathophysiology[edit]
In the body, crystalline silica particles do not dissolve over clinically relevant periods. Silica crystals inside the lungs can activate the NLRP3 inflammasome inside macrophages and dendritic cells and thereby result in production of interleukin, a highly pro-inflammatory cytokine in the immune system.[59][60][61]
Regulation[edit]
Regulations restricting silica exposure ‘with respect to the silicosis hazard’ specify that they are concerned only with silica, which is both crystalline and dust-forming.[62][63][64][65][66][67]
In 2013, the U.S. Occupational Safety and Health Administration reduced the exposure limit to 50 µg/m3 of air. Prior to 2013, it had allowed 100 µg/m3 and in construction workers even 250 µg/m3.[23]
In 2013, OSHA also required «green completion» of fracked wells to reduce exposure to crystalline silica besides restricting the limit of exposure.[23]
Crystalline forms[edit]
SiO2, more so than almost any material, exists in many crystalline forms. These forms are called polymorphs.
| Form | Crystal symmetry Pearson symbol, group No. |
ρ g/cm3 |
Notes | Structure |
|---|---|---|---|---|
| α-quartz | rhombohedral (trigonal) hP9, P3121 No.152[68] |
2.648 | Helical chains making individual single crystals optically active; α-quartz converts to β-quartz at 846 K | 
|
| β-quartz | hexagonal hP18, P6222, No. 180[69] |
2.533 | Closely related to α-quartz (with an Si-O-Si angle of 155°) and optically active; β-quartz converts to β-tridymite at 1140 K | 
|
| α-tridymite | orthorhombic oS24, C2221, No.20[70] |
2.265 | Metastable form under normal pressure | 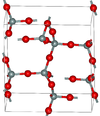
|
| β-tridymite | hexagonal hP12, P63/mmc, No. 194[70] |
Closely related to α-tridymite; β-tridymite converts to β-cristobalite at 2010 K | 
|
|
| α-cristobalite | tetragonal tP12, P41212, No. 92[71] |
2.334 | Metastable form under normal pressure | 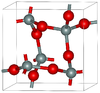
|
| β-cristobalite | cubic cF104, Fd3m, No.227[72] |
Closely related to α-cristobalite; melts at 1978 K | 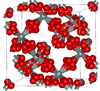
|
|
| keatite | tetragonal tP36, P41212, No. 92[73] |
3.011 | Si5O10, Si4O8, Si8O16 rings; synthesised from glassy silica and alkali at 600–900 K and 40–400 MPa | 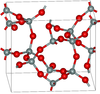
|
| moganite | monoclinic mS46, C2/c, No.15[74] |
Si4O8 and Si6O12 rings | 
|
|
| coesite | monoclinic mS48, C2/c, No.15[75] |
2.911 | Si4O8 and Si8O16 rings; 900 K and 3–3.5 GPa | 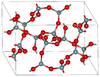
|
| stishovite | tetragonal tP6, P42/mnm, No.136[76] |
4.287 | One of the densest (together with seifertite) polymorphs of silica; rutile-like with 6-fold coordinated Si; 7.5–8.5 GPa | 
|
| seifertite | orthorhombic oP, Pbcn[77] |
4.294 | One of the densest (together with stishovite) polymorphs of silica; is produced at pressures above 40 GPa.[78] | 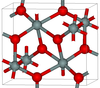
|
| melanophlogite | cubic (cP*, P4232, No.208)[7] or tetragonal (P42/nbc)[79] | 2.04 | Si5O10, Si6O12 rings; mineral always found with hydrocarbons in interstitial spaces — a clathrasil (silica clathrate)[80] | 
|
| fibrous W-silica[11] |
orthorhombic oI12, Ibam, No.72[81] |
1.97 | Like SiS2 consisting of edge sharing chains, melts at ~1700 K | 
|
| 2D silica[82] | hexagonal | Sheet-like bilayer structure | 
|
Safety[edit]
Inhaling finely divided crystalline silica can lead to severe inflammation of the lung tissue, silicosis, bronchitis, lung cancer, and systemic autoimmune diseases, such as lupus and rheumatoid arthritis. Inhalation of amorphous silicon dioxide, in high doses, leads to non-permanent short-term inflammation, where all effects heal.[83]
Other names[edit]
This extended list enumerates synonyms for silicon dioxide; all of these values are from a single source; values in the source were presented capitalized.[84]
- CAS 112945-52-5
- Acitcel
- Aerosil
- Amorphous silica dust
- Aquafil
- CAB-O-GRIP II
- CAB-O-SIL
- CAB-O-SPERSE
- Catalogue
- Colloidal silica[citation needed]
- Colloidal silicon dioxide
- Dicalite
- DRI-DIE Insecticide 67
- FLO-GARD
- Fossil flour
- Fumed silica
- Fumed silicon dioxide
- HI-SEL
- LO-VEL
- Ludox
- Nalcoag
- Nyacol
- Santocel
- Silica
- Silica aerogel
- Silica, amorphous
- Silicic anhydride
- Silikill
- Synthetic amorphous silica
- Vulkasil
See also[edit]
- Mesoporous silica
- Orthosilicic acid
- Silicon carbide
References[edit]
- ^ a b c d e Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1-4398-5511-0.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. «#0552». National Institute for Occupational Safety and Health (NIOSH).
- ^ a b NIOSH Pocket Guide to Chemical Hazards. «#0682». National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ Iler RK (1979). The Chemistry of Silica. New York: Wiley. ISBN 9780471024040.
- ^ a b Fernández LD, Lara E, Mitchell EA (2015). «Checklist, diversity and distribution of testate amoebae in Chile» (PDF). European Journal of Protistology. 51 (5): 409–24. doi:10.1016/j.ejop.2015.07.001. PMID 26340665. Archived (PDF) from the original on 2022-10-10.
- ^ a b Skinner BJ, Appleman DE (1963). «Melanophlogite, a cubic polymorph of silica» (PDF). Am. Mineral. 48: 854–867. Archived (PDF) from the original on 2022-10-10.
- ^ a b c d e f g Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- ^ Cuff YH (1996). Ceramic Technology for Potters and Sculptors. Philadelphia: University of Pennsylvania. pp. 93–95. ISBN 9780812213775.
- ^ De La Rocha C, Conley DJ (2017). «Mystical Crystals of Silica». Silica Stories. Cham: Springer. pp. 50–55. doi:10.1007/978-3-319-54054-2_4. ISBN 9783319540542.
- ^ a b c d e f g h Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 393–99. ISBN 978-0-08-022057-4.
- ^ Wells AF (1984). Structural Inorganic Chemistry. Oxford Science Publications. ISBN 9780198553700.
- ^ Kirfel A, Krane HG, Blaha P, et al. (2001). «Electron-density distribution in stishovite, SiO2: a new high-energy synchrotron-radiation study». Acta Crystallogr. A. 57 (6): 663–77. doi:10.1107/S0108767301010698. PMID 11679696.
- ^ Scherzer J (1978). «Dealuminated faujasite-type structures with SiO2/Al2O3 ratios over 100″. J. Catal. 54 (2): 285. doi:10.1016/0021-9517(78)90051-9.
- ^ Shell SM, Debenedetti PG, Panagiotopoulos AZ (2002). «Molecular structural order and anomalies in liquid silica» (PDF). Phys. Rev. E. 66 (1): 011202. arXiv:cond-mat/0203383. Bibcode:2002PhRvE..66a1202S. doi:10.1103/PhysRevE.66.011202. PMID 12241346. S2CID 6109212. Archived from the original (PDF) on 2016-06-04. Retrieved 2009-07-07.
- ^ Aksay IA, Pask JA, Davis RF (1979). «Densities of SiO2-Al2O3 Melts» (PDF). J. Am. Ceram. Soc. 62 (7–8): 332–336. doi:10.1111/j.1151-2916.1979.tb19071.x. Archived (PDF) from the original on 2022-10-10.
- ^ Jutzi P, Schubert U (2003). Silicon chemistry: from the atom to extended systems. Wiley-VCH. ISBN 9783527306473.
- ^ a b c d e Flörke OW, Graetsch HA, Brunk F, et al. (2018). «Silica». Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_583.pub3.
- ^ Berslien E (2012). An Introduction to Forensic Geoscience. Wiley & Sons. p. 138. ISBN 9781405160544.
- ^ Massey FP, Ennos AR, Hartley SE (2006). «Silica in grasses as a defence against insect herbivores: Contrasting effects on folivores and a phloem feeder». J. Anim. Ecol. 75 (2): 595–603. doi:10.1111/j.1365-2656.2006.01082.x. PMID 16638012.
- ^ Keeping MG, Kvedaras OL (2008). «Silicon as a plant defence against insect herbivory: Response to Massey, Ennos and Hartley». J. Anim. Ecol. 77 (3): 631–3. doi:10.1111/j.1365-2656.2008.01380.x. PMID 18341561.
- ^ Nevin, Charles Merrick (1925). Albany moulding sands of the Hudson Valley. University of the State of New York at Albany.
- ^ a b c d Greenhouse S (23 Aug 2013). «New Rules Would Cut Silica Dust Exposure». The New York Times. Retrieved 24 Aug 2013.
- ^ Ojovan MI (2004). «Glass formation in amorphous SiO2 as a percolation phase transition in a system of network defects». JETP Lett. 79 (12): 632–634. Bibcode:2004JETPL..79..632O. doi:10.1134/1.1790021. S2CID 124299526.
- ^ Elliott SR (1991). «Medium-range structural order in covalent amorphous solids». Nature. 354 (6353): 445–452. Bibcode:1991Natur.354..445E. doi:10.1038/354445a0. S2CID 4344891.
- ^ Atkins PW, Overton T, Rourke J, et al., eds. (2010). Shriver & Atkins’ inorganic chemistry (5th ed.). Oxford: Oxford University Press. p. 354. ISBN 9780199236176. OCLC 430678988.
- ^ a b «Cab-O-Sil Fumed Metal Oxides».
- ^ Cheraghian, Goshtasp; Wistuba, Michael P.; Kiani, Sajad; Barron, Andrew R.; Behnood, Ali (December 2021). «Rheological, physicochemical, and microstructural properties of asphalt binder modified by fumed silica nanoparticles». Scientific Reports. 11 (1): 11455. Bibcode:2021NatSR..1111455C. doi:10.1038/s41598-021-90620-w. PMC 8169902. PMID 34075083.
- ^ Barel AO, Paye M, Maibach HI (2014). Handbook of Cosmetic Science and Technology (4th ed.). CRC Press. p. 444. ISBN 9781842145654.
These soft-focus pigments, mainly composed of polymers, micas and talcs covered with rough or spherical particles of small diameters, such as silica or titanium dioxide, are used to optically reduce the appearance of wrinkles. These effects are obtained by optimizing outlines of wrinkles and reducing the difference of brightness due to diffuse reflection.
- ^ Barel AO, Paye M, Maibach HI (2014). Handbook of Cosmetic Science and Technology (4th ed.). CRC Press. p. 442. ISBN 9781842145654.
The silica is a multiporous ingredient, which absorbs the oil and sebum.
- ^ Bassett, Ross Knox (2007). To the Digital Age: Research Labs, Start-up Companies, and the Rise of MOS Technology. Johns Hopkins University Press. pp. 22–23. ISBN 9780801886393.
- ^ a b Lécuyer, Christophe; Brock, David C. (2010). Makers of the Microchip: A Documentary History of Fairchild Semiconductor. MIT Press. p. 111. ISBN 9780262294324.
- ^ a b Saxena, A (2009). Invention of integrated circuits: untold important facts. International series on advances in solid state electronics and technology. World Scientific. pp. 96–97. ISBN 9789812814456.
- ^ a b c «Martin Atalla in Inventors Hall of Fame, 2009». Retrieved 21 June 2013.
- ^ a b Black, Lachlan E. (2016). New Perspectives on Surface Passivation: Understanding the Si-Al2O3 Interface. Springer. p. 17. ISBN 9783319325217.
- ^ a b «Dawon Kahng». National Inventors Hall of Fame. Retrieved 27 June 2019.
- ^ Goodwin W, Linacre A, Hadi S (2007). An Introduction to Forensic Genetics. Wiley & Sons. p. 29. ISBN 9780470010259.
- ^ Calderone J (20 Aug 2015). «This cloud-like, futuristic material has been sneaking its way into your life since 1931». Business Insider. Retrieved 11 Feb 2019.
- ^ Thabet, Ahmed F.; Boraei, Hessien A.; Galal, Ola A.; El-Samahy, Magdy F. M.; Mousa, Kareem M.; Zhang, Yao Z.; Tuda, Midori; Helmy, Eman A.; Wen, Jian; Nozaki, Tsubasa (2021-07-14). «Silica nanoparticles as pesticide against insects of different feeding types and their non-target attraction of predators». Scientific Reports. 11 (1): 14484. Bibcode:2021NatSR..1114484T. doi:10.1038/s41598-021-93518-9. ISSN 2045-2322. PMC 8280210. PMID 34262071.
- ^ a b Meyer A, Sandler DP, Beane Freeman LE, et al. (2017). «Pesticide Exposure and Risk of Rheumatoid Arthritis among Licensed Male Pesticide Applicators in the Agricultural Health Study». Environmental Health Perspectives. 125 (7): 077010-1–077010-7. doi:10.1289/EHP1013. PMC 5744649. PMID 28718769.
- ^ Doering R, Nishi Y, eds. (2007). Handbook of Semiconductor Manufacturing Technology. CRC Press. ISBN 9781574446753.
- ^ Lee S (2006). Encyclopedia of chemical processing. CRC Press. ISBN 9780824755638.
- ^ Morgan DV, Board K (1991). An Introduction To Semiconductor Microtechnology (2nd ed.). Chichester, West Sussex, England: John Wiley & Sons. p. 72. ISBN 9780471924784.
- ^ Riordan M (2007). «The Silicon Dioxide Solution: How physicist Jean Hoerni built the bridge from the transistor to the integrated circuit». IEEE Spectrum. Retrieved 11 Feb 2019.
- ^ Chrystie, Robin S. M.; Ebertz, Felix L.; Dreier, Thomas; Schulz, Christof (2019-01-28). «Absolute SiO concentration imaging in low-pressure nanoparticle-synthesis flames via laser-induced fluorescence». Applied Physics B. 125 (2): 29. Bibcode:2019ApPhB.125…29C. doi:10.1007/s00340-019-7137-8. ISSN 1432-0649. S2CID 127735545.
- ^ Romero-Jaime, A. K.; Acosta-Enríquez, M. C.; Vargas-Hernández, D.; Tánori-Córdova, J. C.; Pineda León, H. A.; Castillo, S. J. (August 2021). «Synthesis and characterization of silica–lead sulfide core–shell nanospheres for applications in optoelectronic devices». Journal of Materials Science: Materials in Electronics. 32 (16): 21425–21431. doi:10.1007/s10854-021-06648-1. ISSN 0957-4522. S2CID 236182027.
- ^ Nandiyanto AB, Kim SG, Iskandar F, et al. (2009). «Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters». Microporous and Mesoporous Materials. 120 (3): 447–453. doi:10.1016/j.micromeso.2008.12.019.
- ^ Morgan DV, Board K (1991). An Introduction To Semiconductor Microtechnology (2nd ed.). Chichester, West Sussex, England: John Wiley & Sons. p. 27. ISBN 9780471924784.
- ^ Fleischer, Michael (1962). «New mineral names» (PDF). American Mineralogist. Mineralogical Society of America. 47 (2): 172–174. Archived (PDF) from the original on 2011-07-22.
- ^ Rodgers GE (2011). Descriptive Inorganic, Coordination, and Solid State Chemistry. Cengage Learning. pp. 421–2. ISBN 9781133172482.
- ^ Laine, Richard M.; Blohowiak, Kay Youngdahl; Robinson, Timothy R.; Hoppe, Martin L.; Nardi, Paola; Kampf, Jeffrey; Uhm, Jackie (17 October 1991). «Synthesis of pentacoordinate silicon complexes from SiO2» (PDF). Nature. 353 (6345): 642–644. Bibcode:1991Natur.353..642L. doi:10.1038/353642a0. hdl:2027.42/62810. S2CID 4310228. Archived (PDF) from the original on 2017-08-19.
- ^ Fournier RO, Rowe JJ (1977). «The solubility of amorphous silica in water at high temperatures and high pressures» (PDF). Am. Mineral. 62: 1052–1056. Archived (PDF) from the original on 2022-10-10.
- ^ Okamoto, Atsushi (2019). «Formation of silica particles from supercritical fluids and its impacts on the hydrological properties in the crust». EGU General Assembly Conference Abstracts: 4614. Bibcode:2019EGUGA..21.4614O.
- ^ Rondeau V, Jacqmin-Gadda H, Commenges D, et al. (2008). «Aluminum and Silica in Drinking Water and the Risk of Alzheimer’s Disease or Cognitive Decline: Findings from 15-Year Follow-up of the PAQUID Cohort». American Journal of Epidemiology. 169 (4): 489–96. doi:10.1093/aje/kwn348. PMC 2809081. PMID 19064650.
- ^ «Work Safely with Silica». CPWR — The Center for Construction Research and Training. Retrieved 11 Feb 2019.
- ^ «Action Plan for Lupus Research». National Institute of Arthritis and Musculoskeletal and Skin Diseases. National Institutes of Health. 2017. Retrieved 11 Feb 2019.
- ^ Reuzel PG, Bruijntjes JP, Feron VJ, et al. (1991). «Subchronic inhalation toxicity of amorphous silica and quartz dust in rats». Food Chem. Toxicol. 29 (5): 341–54. doi:10.1016/0278-6915(91)90205-L. PMID 1648030.
- ^ «Worker Exposure to Silica during Countertop Manufacturing, Finishing and Installation» (PDF). National Institute for Occupational Safety and Health and Occupational Safety and Health Administration. 2015. Archived (PDF) from the original on 2022-10-10. Retrieved 26 Feb 2015.
- ^ Hornung V, Bauernfeind F, Halle A, et al. (2008). «Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization». Nat. Immunol. 9 (8): 847–856. doi:10.1038/ni.1631. PMC 2834784. PMID 18604214.
- ^ Merchant JA, ed. (1986). Occupational Respiratory Diseases (PDF). Cincinnati, OH: US Department of Health and Human Services, NIOSH. doi:10.26616/NIOSHPUB86102. hdl:2027/uc1.31210023588922. DHHS (NIOSH) Publication Number 86-102.
- ^ NIOSH (2002) Hazard Review, Health Effects of Occupational Exposure to Respirable Crystalline Silica. Cincinnati, OH: U.S. Department of Health and Human Services, U.S. Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2002-129.
- ^ «Crystalline Factsheet» (PDF). Archived from the original (PDF) on 22 December 2017. Retrieved 3 August 2017.
- ^ «Silica, Crystalline». Retrieved 3 August 2017.
- ^ «Frequently Asked Questions». Retrieved 3 August 2017.
- ^ «If It’s Silica, It’s Not Just Dust!» (PDF). Archived (PDF) from the original on 2022-10-10. Retrieved 3 August 2017.
- ^ «What you should know about crystalline silica, silicosis, and Oregon OSHA silica rules» (PDF). Archived (PDF) from the original on 2022-10-10. Retrieved 3 August 2017.
- ^ Szymendera, Scott D. (January 16, 2018). Respirable Crystalline Silica in the Workplace: New Occupational Safety and Health Administration (OSHA) Standards (PDF). Washington, DC: Congressional Research Service. Archived (PDF) from the original on 2022-10-10. Retrieved 27 January 2018.
- ^ Lager G. A.; Jorgensen J. D.; Rotella F.J. (1982). «Crystal structure and thermal expansion of a-quartz SiO2 at low temperature». Journal of Applied Physics. 53 (10): 6751–6756. Bibcode:1982JAP….53.6751L. doi:10.1063/1.330062.
- ^ Wright, A. F.; Lehmann, M. S. (1981). «The structure of quartz at 25 and 590 °C determined by neutron diffraction». Journal of Solid State Chemistry. 36 (3): 371–80. Bibcode:1981JSSCh..36..371W. doi:10.1016/0022-4596(81)90449-7.
- ^ a b Kihara, Kuniaki; Matsumoto, Takeo; Imamura, Moritaka (1986). «Structural change of orthorhombic-Itridymite with temperature: A study based on second-order thermal-vibrational parameters». Zeitschrift für Kristallographie. 177 (1–2): 27–38. Bibcode:1986ZK….177…27K. doi:10.1524/zkri.1986.177.1-2.27.
- ^ Downs R. T.; Palmer D. C. (1994). «The pressure behavior of a cristobalite» (PDF). American Mineralogist. 79: 9–14. Archived (PDF) from the original on 2022-10-10.
- ^ Wright, A. F.; Leadbetter, A. J. (1975). «The structures of the β-cristobalite phases of SiO2 and AlPO4«. Philosophical Magazine. 31 (6): 1391–401. Bibcode:1975PMag…31.1391W. doi:10.1080/00318087508228690.
- ^ Shropshire, Joseph; Keat, Paul P.; Vaughan, Philip A. (1959). «The crystal structure of keatite, a new form of silica». Zeitschrift für Kristallographie. 112 (1–6): 409–13. Bibcode:1959ZK….112..409S. doi:10.1524/zkri.1959.112.1-6.409.
- ^ Miehe, Gerhard; Graetsch, Heribert (1992). «Crystal structure of moganite: a new structure type for silica». European Journal of Mineralogy. 4 (4): 693–706. Bibcode:1992EJMin…4..693M. doi:10.1127/ejm/4/4/0693.
- ^ Levien L.; Prewitt C. T. (1981). «High-pressure crystal structure and compressibility of coesite» (PDF). American Mineralogist. 66: 324–333. Archived (PDF) from the original on 2022-10-10.
- ^ Smyth J. R.; Swope R. J.; Pawley A. R. (1995). «H in rutile-type compounds: II. Crystal chemistry of Al substitution in H-bearing stishovite» (PDF). American Mineralogist. 80 (5–6): 454–456. Bibcode:1995AmMin..80..454S. doi:10.2138/am-1995-5-605. S2CID 196903109. Archived (PDF) from the original on 2022-10-10.
- ^ Dera P.; Prewitt C. T.; Boctor N. Z.; Hemley R. J. (2002). «Characterization of a high-pressure phase of silica from the Martian meteorite Shergotty». American Mineralogist. 87 (7): 1018. Bibcode:2002AmMin..87.1018D. doi:10.2138/am-2002-0728. S2CID 129400258.
- ^ Seifertite. Mindat.org.
- ^ Nakagawa T.; Kihara K.; Harada K. (2001). «The crystal structure of low melanophlogite». American Mineralogist. 86 (11–12): 1506. Bibcode:2001AmMin..86.1506N. doi:10.2138/am-2001-11-1219. S2CID 53525827.
- ^ Rosemarie Szostak (1998). Molecular sieves: Principles of Synthesis and Identification. Springer. ISBN 978-0-7514-0480-7.
- ^ Weiss, Alarich; Weiss, Armin (1954). «Über Siliciumchalkogenide. VI. Zur Kenntnis der faserigen Siliciumdioxyd-Modifikation». Zeitschrift für Anorganische und Allgemeine Chemie. 276 (1–2): 95–112. doi:10.1002/zaac.19542760110.
- ^ Björkman, T; Kurasch, S; Lehtinen, O; Kotakoski, J; Yazyev, O. V.; Srivastava, A; Skakalova, V; Smet, J. H.; Kaiser, U; Krasheninnikov, A. V. (2013). «Defects in bilayer silica and graphene: common trends in diverse hexagonal two-dimensional systems». Scientific Reports. 3: 3482. Bibcode:2013NatSR…3E3482B. doi:10.1038/srep03482. PMC 3863822. PMID 24336488.
- ^ Johnston CJ, Driscoll KE, Finkelstein JN, et al. (2000). «Pulmonary Chemokine and Mutagenic Responses in Rats after Subchronic Inhalation of Amorphous and Crystalline Silica». Toxicological Sciences. 56 (2): 405–413. doi:10.1093/toxsci/56.2.405. PMID 10911000.
- ^ Lewis, Grace Ross (1999). 1001 chemicals in everyday products (2nd ed.). John Wiley & Sons (Wiley-Interscience). pp. 250–1. ISBN 0-471-29212-5 – via Internet Archive.
External links[edit]
- Chisholm, Hugh, ed. (1911). «Silica» . Encyclopædia Britannica (11th ed.). Cambridge University Press.
- Tridymite, International Chemical Safety Card 0807
- Quartz, International Chemical Safety Card 0808
- Cristobalite, International Chemical Safety Card 0809
- Amorphous, NIOSH Pocket Guide to Chemical Hazards
- Crystalline, as respirable dust, NIOSH Pocket Guide to Chemical Hazards
- Formation of silicon oxide layers in the semiconductor industry. LPCVD and PECVD method in comparison. Stress prevention.
- Quartz (SiO2) piezoelectric properties
- Silica (SiO2) and water
- Epidemiological evidence on the carcinogenicity of silica: factors in scientific judgement by C. Soutar and others. Institute of Occupational Medicine Research Report TM/97/09
- Scientific opinion on the health effects of airborne silica by A Pilkington and others. Institute of Occupational Medicine Research Report TM/95/08
- The toxic effects of silica Archived 2016-04-15 at the Wayback Machine by A. Seaton and others. Institute of Occupational Medicine Research Report TM/87/13
- Structure of precipitated silica
A sample of silicon dioxide |
|
| Names | |
|---|---|
| IUPAC name
Silicon dioxide |
|
Other names
|
|
| Identifiers | |
|
CAS Number |
|
| ChEBI |
|
| ChemSpider |
|
| ECHA InfoCard | 100.028.678 |
| EC Number |
|
| E number | E551 (acidity regulators, …) |
|
Gmelin Reference |
200274 |
| KEGG |
|
| MeSH | Silicon+dioxide |
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
|
CompTox Dashboard (EPA) |
|
|
InChI
|
|
| Properties | |
|
Chemical formula |
SiO2 |
| Molar mass | 60.08 g/mol |
| Appearance | Transparent solid (Amorphous) White/Whitish Yellow (Powder/Sand) |
| Density | 2.648 (α-quartz), 2.196 (amorphous) g·cm−3[1] |
| Melting point | 1,713 °C (3,115 °F; 1,986 K) (amorphous)[1]: 4.88 to |
| Boiling point | 2,950 °C (5,340 °F; 3,220 K)[1] |
|
Magnetic susceptibility (χ) |
−29.6·10−6 cm3/mol |
| Thermal conductivity | 12 (|| c-axis), 6.8 (⊥ c-axis), 1.4 (am.) W/(m⋅K)[1]: 12.213 |
|
Refractive index (nD) |
1.544 (o), 1.553 (e)[1]: 4.143 |
| Hazards | |
| NFPA 704 (fire diamond) |
0 0 0 |
| NIOSH (US health exposure limits): | |
|
PEL (Permissible) |
TWA 20 mppcf (80 mg/m3/%SiO2) (amorphous)[2] |
|
REL (Recommended) |
TWA 6 mg/m3 (amorphous)[2] Ca TWA 0.05 mg/m3[3] |
|
IDLH (Immediate danger) |
3000 mg/m3 (amorphous)[2] Ca [25 mg/m3 (cristobalite, tridymite); 50 mg/m3 (quartz)][3] |
| Related compounds | |
|
Related diones |
Carbon dioxide
Germanium dioxide |
|
Related compounds |
Silicon monoxide
Silicon sulfide |
| Thermochemistry | |
|
Std molar |
42 J·mol−1·K−1[4] |
|
Std enthalpy of |
−911 kJ·mol−1[4] |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, most commonly found in nature as quartz.[5][6] In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an electrical insulator), and as components in the food and pharmaceutical industries.
Structure[edit]
Structural motif found in α-quartz, but also found in almost all forms of silicon dioxide
Typical subunit for low pressure silicon dioxide
Relationship between refractive index and density for some SiO2 forms[7]
In the majority of silicon dioxides, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atom (see 3-D Unit Cell). Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear molecule. The starkly different structures of the dioxides of carbon and silicon are a manifestation of the double bond rule.
SiO2 has several distinct crystalline forms, but they almost always have the same local structure around Si and O. In α-quartz the Si–O bond length is 161 pm, whereas in α-tridymite it is in the range 154–171 pm. The Si–O–Si angle also varies between a low value of 140° in α-tridymite, up to 180° in β-tridymite. In α-quartz, the Si–O–Si angle is 144°.[8]
Polymorphism[edit]
Alpha quartz is the most stable form of solid SiO2 at room temperature. The high-temperature minerals, cristobalite and tridymite, have both lower densities and indices of refraction than quartz. The transformation from α-quartz to beta-quartz takes place abruptly at 573 °C. Since the transformation is accompanied by a significant change in volume, it can easily induce fracturing of ceramics or rocks passing through this temperature limit.[9] The high-pressure minerals, seifertite, stishovite, and coesite, though, have higher densities and indices of refraction than quartz.[10] Stishovite has a rutile-like structure where silicon is 6-coordinate. The density of stishovite is 4.287 g/cm3, which compares to α-quartz, the densest of the low-pressure forms, which has a density of 2.648 g/cm3.[11] The difference in density can be ascribed to the increase in coordination as the six shortest Si–O bond lengths in stishovite (four Si–O bond lengths of 176 pm and two others of 181 pm) are greater than the Si–O bond length (161 pm) in α-quartz.[12]
The change in the coordination increases the ionicity of the Si–O bond.[13] More importantly, any deviations from these standard parameters constitute microstructural differences or variations, which represent an approach to an amorphous, vitreous, or glassy solid.
Faujasite silica, another polymorph, is obtained by dealumination of a low-sodium, ultra-stable Y zeolite with combined acid and thermal treatment. The resulting product contains over 99% silica, and has high crystallinity and specific surface area (over 800 m2/g). Faujasite-silica has very high thermal and acid stability. For example, it maintains a high degree of long-range molecular order or crystallinity even after boiling in concentrated hydrochloric acid.[14]
Molten SiO2[edit]
Molten silica exhibits several peculiar physical characteristics that are similar to those observed in liquid water: negative temperature expansion, density maximum at temperatures ~5000 °C, and a heat capacity minimum.[15] Its density decreases from 2.08 g/cm3 at 1950 °C to 2.03 g/cm3 at 2200 °C.[16]
Molecular SiO2[edit]
The molecular SiO2 has a linear structure like CO2. It has been produced by combining silicon monoxide (SiO) with oxygen in an argon matrix.
The dimeric silicon dioxide, (SiO2)2 has been obtained by reacting O2 with matrix isolated dimeric silicon monoxide, (Si2O2). In dimeric silicon dioxide there are two oxygen atoms bridging between the silicon atoms with an Si–O–Si angle of 94° and bond length of 164.6 pm and the terminal Si–O bond length is 150.2 pm. The Si–O bond length is 148.3 pm, which compares with the length of 161 pm in α-quartz. The bond energy is estimated at 621.7 kJ/mol.[17]
Natural occurrence[edit]
Geology[edit]
SiO2 is most commonly found in nature as quartz, which comprises more than 10% by mass of the Earth’s crust.[18] Quartz is the only polymorph of silica stable at the Earth’s surface. Metastable occurrences of the high-pressure forms coesite and stishovite have been found around impact structures and associated with eclogites formed during ultra-high-pressure metamorphism. The high-temperature forms of tridymite and cristobalite are known from silica-rich volcanic rocks. In many parts of the world, silica is the major constituent of sand.[19]
Biology[edit]
Even though it is poorly soluble, silica occurs in many plants such as rice. Plant materials with high silica phytolith content appear to be of importance to grazing animals, from chewing insects to ungulates. Silica accelerates tooth wear, and high levels of silica in plants frequently eaten by herbivores may have developed as a defense mechanism against predation.[20][21]
Silica is also the primary component of rice husk ash, which is used, for example, in filtration and as supplementary cementitious material (SCM) in cement and concrete manufacturing.[citation needed]
For well over a 1000 million years, silicification in and by cells has been common in the biological world. In the modern world, it occurs in bacteria, single-celled organisms, plants, and animals (invertebrates and vertebrates).
Prominent examples include:
- Tests or frustules (i.e. shells) of diatoms, Radiolaria, and testate amoebae.[6]
- Silica phytoliths in the cells of many plants, including Equisetaceae, practically all grasses, and a wide range of dicotyledons.
- The spicules forming the skeleton of many sponges.
Crystalline minerals formed in the physiological environment often show exceptional physical properties (e.g., strength, hardness, fracture toughness) and tend to form hierarchical structures that exhibit microstructural order over a range of scales. The minerals are crystallized from an environment that is undersaturated concerning silicon, and under conditions of neutral pH and low temperature (0–40 °C).
Uses[edit]
Structural use[edit]
About 95% of the commercial use of silicon dioxide (sand) occurs in the construction industry, e.g. for the production of concrete (Portland cement concrete).[18]
Certain deposits of silica sand, with desirable particle size and shape and desirable clay and other mineral content, were important for sand casting of metallic products.[22] The high melting point of silica enables it to be used in such applications such as iron casting; modern sand casting sometimes uses other minerals for other reasons.
Crystalline silica is used in hydraulic fracturing of formations which contain tight oil and shale gas.[23]
Precursor to glass and silicon[edit]
Silica is the primary ingredient in the production of most glass. As other minerals are melted with silica, the principle of freezing point depression lowers the melting point of the mixture and increases fluidity. The glass transition temperature of pure SiO2 is about 1475 K.[24] When molten silicon dioxide SiO2 is rapidly cooled, it does not crystallize, but solidifies as a glass. Because of this, most ceramic glazes have silica as the main ingredient.
The structural geometry of silicon and oxygen in glass is similar to that in quartz and most other crystalline forms of silicon and oxygen with silicon surrounded by regular tetrahedra of oxygen centres. The difference between the glass and crystalline forms arises from the connectivity of the tetrahedral units: Although there is no long-range periodicity in the glassy network ordering remains at length scales well beyond the SiO bond length. One example of this ordering is the preference to form rings of 6-tetrahedra.[25]
The majority of optical fibers for telecommunication are also made from silica. It is a primary raw material for many ceramics such as earthenware, stoneware, and porcelain.
Silicon dioxide is used to produce elemental silicon. The process involves carbothermic reduction in an electric arc furnace:[26]
Fumed silica[edit]
Fumed silica, also known as pyrogenic silica, is prepared by burning SiCl4 in an oxygen-rich hydrogen flame to produce a «smoke» of SiO2.[11]
It can also be produced by vaporizing quartz sand in a 3000 °C electric arc. Both processes result in microscopic droplets of amorphous silica fused into branched, chainlike, three-dimensional secondary particles which then agglomerate into tertiary particles, a white powder with extremely low bulk density (0.03-.15 g/cm3) and thus high surface area.[27] The particles act as a thixotropic thickening agent, or as an anti-caking agent, and can be treated to make them hydrophilic or hydrophobic for either water or organic liquid applications
Manufactured fumed silica with maximum surface area of 380 m2/g
Silica fume is an ultrafine powder collected as a by-product of the silicon and ferrosilicon alloy production. It consists of amorphous (non-crystalline) spherical particles with an average particle diameter of 150 nm, without the branching of the pyrogenic product. The main use is as pozzolanic material for high performance concrete. Fumed silica nanoparticles can be successfully used as an anti-aging agent in asphalt binders.[28]
Food, cosmetic, and pharmaceutical applications[edit]
Silica, either colloidal, precipitated, or pyrogenic fumed, is a common additive in food production. It is used primarily as a flow or anti-caking agent in powdered foods such as spices and non-dairy coffee creamer, or powders to be formed into pharmaceutical tablets.[27] It can adsorb water in hygroscopic applications. Colloidal silica is used as a fining agent for wine, beer, and juice, with the E number reference E551.[18]
In cosmetics, silica is useful for its light-diffusing properties[29] and natural absorbency.[30]
Diatomaceous earth, a mined product, has been used in food and cosmetics for centuries. It consists of the silica shells of microscopic diatoms; in a less processed form it was sold as «tooth powder».[citation needed] Manufactured or mined hydrated silica is used as the hard abrasive in toothpaste.
Semiconductors[edit]
Silicon dioxide is widely used in the semiconductor technology
- for the primary passivation (directly on the semiconductor surface),
- as an original gate dielectric in MOS technology. Today when scaling (dimension of the gate length of the MOS transistor) has progressed below 10 nm silicon dioxide has been replaced by other dielectric materials like hafnium oxide or similar with higher dielectric constant compared to silicon dioxide,
- as a dielectric layer between metal (wiring) layers (sometimes up to 8-10) connecting elements and
- as a second passivation layer (for protecting semiconductor elements and the metallization layers) typically today layered with some other dielectrics like silicon nitride.
Because silicon dioxide is a native oxide of silicon it is more widely used compared to other semiconductors like gallium arsenide or indium phosphide.
Silicon dioxide could be grown on a silicon semiconductor surface.[31] Silicon oxide layers could protect silicon surfaces during diffusion processes, and could be used for diffusion masking.[32][33]
Surface passivation is the process by which a semiconductor surface is rendered inert, and does not change semiconductor properties as a result of interaction with air or other materials in contact with the surface or edge of the crystal.[34][35] The formation of a thermally grown silicon dioxide layer greatly reduces the concentration of electronic states at the silicon surface.[35] SiO2 films preserve the electrical characteristics of p–n junctions and prevent these electrical characteristics from deteriorating by the gaseous ambient environment.[33] Silicon oxide layers could be used to electrically stabilize silicon surfaces.[32] The surface passivation process is an important method of semiconductor device fabrication that involves coating a silicon wafer with an insulating layer of silicon oxide so that electricity could reliably penetrate to the conducting silicon below. Growing a layer of silicon dioxide on top of a silicon wafer enables it to overcome the surface states that otherwise prevent electricity from reaching the semiconducting layer.[34][36]
The process of silicon surface passivation by thermal oxidation (silicon dioxide) is critical to the semiconductor industry. It is commonly used to manufacture metal–oxide–semiconductor field-effect transistors (MOSFETs) and silicon integrated circuit chips (with the planar process).[34][36]
Other[edit]
Hydrophobic silica is used as a defoamer component.
In its capacity as a refractory, it is useful in fiber form as a high-temperature thermal protection fabric.[citation needed]
Silica is used in the extraction of DNA and RNA due to its ability to bind to the nucleic acids under the presence of chaotropes.[37]
Silica aerogel was used in the Stardust spacecraft to collect extraterrestrial particles.[38]
Pure silica (silicon dioxide), when cooled as fused quartz into a glass with no true melting point, can be used as a glass fibre for fibreglass.
Insecticide[edit]
Silicon dioxide has been researched for agricultural applications as a potential insecticide.[39][40]
Production[edit]
Silicon dioxide is mostly obtained by mining, including sand mining and purification of quartz.
Quartz is suitable for many purposes, while chemical processing is required to make a purer or otherwise more suitable (e.g. more reactive or fine-grained) product.[citation needed]
Precipitated silica[edit]
Precipitated silica or amorphous silica is produced by the acidification of solutions of sodium silicate. The gelatinous precipitate or silica gel, is first washed and then dehydrated to produce colorless microporous silica.[11] The idealized equation involving a trisilicate and sulfuric acid is:
Approximately one billion kilograms/year (1999) of silica were produced in this manner, mainly for use for polymer composites – tires and shoe soles.[18]
On microchips[edit]
Thin films of silica grow spontaneously on silicon wafers via thermal oxidation, producing a very shallow layer of about 1 nm or 10 Å of so-called native oxide.[41]
Higher temperatures and alternative environments are used to grow well-controlled layers of silicon dioxide on silicon, for example at temperatures between 600 and 1200 °C, using so-called dry oxidation with O2
or wet oxidation with H2O.[42][43]
The native oxide layer is beneficial in microelectronics, where it acts as electric insulator with high chemical stability. It can protect the silicon, store charge, block current, and even act as a controlled pathway to limit current flow.[44]
Laboratory or special methods[edit]
From organosilicon compounds[edit]
Many routes to silicon dioxide start with an organosilicon compound, e.g., HMDSO,[45] TEOS. Synthesis of silica is illustrated below using tetraethyl orthosilicate (TEOS).[46] Simply heating TEOS at 680–730 °C results in the oxide:
Similarly TEOS combusts around 400 °C:
TEOS undergoes hydrolysis via the so-called sol-gel process. The course of the reaction and nature of the product are affected by catalysts, but the idealized equation is:[47]
Other methods[edit]
Being highly stable, silicon dioxide arises from many methods. Conceptually simple, but of little practical value, combustion of silane gives silicon dioxide. This reaction is analogous to the combustion of methane:
However the chemical vapor deposition of silicon dioxide onto crystal surface from silane had been used using nitrogen as a carrier gas at 200–500 °C.[48]
Chemical reactions[edit]
Silicon dioxide is a relatively inert material (hence its widespread occurrence as a mineral. Silica is often used as inert containers for chemical reactions. At high temperatures, it is converted to silicon by reduction with carbon.
Fluorine reacts with silicon dioxide to form SiF4 and O2 whereas the other halogen gases (Cl2, Br2, I2) are unreactive.[11]
Most forms of silicon dioxide are attacked («etched») by hydrofluoric acid (HF) to produce hexafluorosilicic acid:[8]
- SiO2 + 6 HF → H2SiF6 + 2 H2O
Stishovite, which does not react to any significant degree[49])
HF is used to remove or pattern silicon dioxide in the semiconductor industry.
Silicon dioxide acts as a Lux–Flood acid, being able to react with bases under certain conditions. As it does not contain any hydrogen, non-hydrated silica cannot directly act as a Brønsted–Lowry acid. While silicon dioxide is only poorly soluble in water at low or neutral pH (typically, 2 × 10−4 M for quartz up to 10−3 M for cryptocrystalline chalcedony), strong bases react with glass and easily dissolve it. Therefore, strong bases have to be stored in plastic bottles to avoid jamming the bottle cap, to preserve the integrity of the recipient, and to avoid undesirable contamination by silicate anions.[50]
Silicon dioxide dissolves in hot concentrated alkali or fused hydroxide, as described in this idealized equation:[11]
Silicon dioxide will neutralise basic metal oxides (e.g. sodium oxide, potassium oxide, lead(II) oxide, zinc oxide, or mixtures of oxides, forming silicates and glasses as the Si-O-Si bonds in silica are broken successively).[8] As an example the reaction of sodium oxide and SiO2 can produce sodium orthosilicate, sodium silicate, and glasses, dependent on the proportions of reactants:[11]
.
Examples of such glasses have commercial significance, e.g. soda-lime glass, borosilicate glass, lead glass. In these glasses, silica is termed the network former or lattice former.[8] The reaction is also used in blast furnaces to remove sand impurities in the ore by neutralisation with calcium oxide, forming calcium silicate slag.
Silicon dioxide reacts in heated reflux under dinitrogen with ethylene glycol and an alkali metal base to produce highly reactive, pentacoordinate silicates which provide access to a wide variety of new silicon compounds.[51] The silicates are essentially insoluble in all polar solvent except methanol.
Silicon dioxide reacts with elemental silicon at high temperatures to produce SiO:[8]
Water solubility[edit]
The solubility of silicon dioxide in water strongly depends on its crystalline form and is three-four times higher for silica[clarification needed] than quartz; as a function of temperature, it peaks around 340 °C (644 °F).[52] This property is used to grow single crystals of quartz in a hydrothermal process where natural quartz is dissolved in superheated water in a pressure vessel that is cooler at the top. Crystals of 0.5–1 kg can be grown for 1–2 months.[8] These crystals are a source of very pure quartz for use in electronic applications.[11] Above the critical temperature of water 647.096 K (373.946 °C; 705.103 °F) and a pressure of 22.064 megapascals (3,200.1 psi) or higher, water is a supercritical fluid and solubility is once again higher than at lower temperatures.[53]
Health effects[edit]
Quartz sand (silica) as main raw material for commercial glass production
Silica ingested orally is essentially nontoxic, with an LD50 of 5000 mg/kg (5 g/kg).[18] A 2008 study following subjects for 15 years found that higher levels of silica in water appeared to decrease the risk of dementia. An increase of 10 mg/day of silica in drinking water was associated with a decreased risk of dementia of 11%.[54]
Inhaling finely divided crystalline silica dust can lead to silicosis, bronchitis, or lung cancer, as the dust becomes lodged in the lungs and continuously irritates the tissue, reducing lung capacities.[55] When fine silica particles are inhaled in large enough quantities (such as through occupational exposure), it increases the risk of systemic autoimmune diseases such as lupus[56] and rheumatoid arthritis compared to expected rates in the general population.[40]
Occupational hazard[edit]
Silica is an occupational hazard for people who do sandblasting or work with products that contain powdered crystalline silica. Amorphous silica, such as fumed silica, may cause irreversible lung damage in some cases but is not associated with the development of silicosis. Children, asthmatics of any age, those with allergies, and the elderly (all of whom have reduced lung capacity) can be affected in less time.[57]
Crystalline silica is an occupational hazard for those working with stone countertops, because the process of cutting and installing the countertops creates large amounts of airborne silica.[58] Crystalline silica used in hydraulic fracturing presents a health hazard to workers.[23]
Pathophysiology[edit]
In the body, crystalline silica particles do not dissolve over clinically relevant periods. Silica crystals inside the lungs can activate the NLRP3 inflammasome inside macrophages and dendritic cells and thereby result in production of interleukin, a highly pro-inflammatory cytokine in the immune system.[59][60][61]
Regulation[edit]
Regulations restricting silica exposure ‘with respect to the silicosis hazard’ specify that they are concerned only with silica, which is both crystalline and dust-forming.[62][63][64][65][66][67]
In 2013, the U.S. Occupational Safety and Health Administration reduced the exposure limit to 50 µg/m3 of air. Prior to 2013, it had allowed 100 µg/m3 and in construction workers even 250 µg/m3.[23]
In 2013, OSHA also required «green completion» of fracked wells to reduce exposure to crystalline silica besides restricting the limit of exposure.[23]
Crystalline forms[edit]
SiO2, more so than almost any material, exists in many crystalline forms. These forms are called polymorphs.
| Form | Crystal symmetry Pearson symbol, group No. |
ρ g/cm3 |
Notes | Structure |
|---|---|---|---|---|
| α-quartz | rhombohedral (trigonal) hP9, P3121 No.152[68] |
2.648 | Helical chains making individual single crystals optically active; α-quartz converts to β-quartz at 846 K | 
|
| β-quartz | hexagonal hP18, P6222, No. 180[69] |
2.533 | Closely related to α-quartz (with an Si-O-Si angle of 155°) and optically active; β-quartz converts to β-tridymite at 1140 K | 
|
| α-tridymite | orthorhombic oS24, C2221, No.20[70] |
2.265 | Metastable form under normal pressure | 
|
| β-tridymite | hexagonal hP12, P63/mmc, No. 194[70] |
Closely related to α-tridymite; β-tridymite converts to β-cristobalite at 2010 K | 
|
|
| α-cristobalite | tetragonal tP12, P41212, No. 92[71] |
2.334 | Metastable form under normal pressure | 
|
| β-cristobalite | cubic cF104, Fd3m, No.227[72] |
Closely related to α-cristobalite; melts at 1978 K | 
|
|
| keatite | tetragonal tP36, P41212, No. 92[73] |
3.011 | Si5O10, Si4O8, Si8O16 rings; synthesised from glassy silica and alkali at 600–900 K and 40–400 MPa | 
|
| moganite | monoclinic mS46, C2/c, No.15[74] |
Si4O8 and Si6O12 rings | 
|
|
| coesite | monoclinic mS48, C2/c, No.15[75] |
2.911 | Si4O8 and Si8O16 rings; 900 K and 3–3.5 GPa | 
|
| stishovite | tetragonal tP6, P42/mnm, No.136[76] |
4.287 | One of the densest (together with seifertite) polymorphs of silica; rutile-like with 6-fold coordinated Si; 7.5–8.5 GPa | 
|
| seifertite | orthorhombic oP, Pbcn[77] |
4.294 | One of the densest (together with stishovite) polymorphs of silica; is produced at pressures above 40 GPa.[78] | 
|
| melanophlogite | cubic (cP*, P4232, No.208)[7] or tetragonal (P42/nbc)[79] | 2.04 | Si5O10, Si6O12 rings; mineral always found with hydrocarbons in interstitial spaces — a clathrasil (silica clathrate)[80] | 
|
| fibrous W-silica[11] |
orthorhombic oI12, Ibam, No.72[81] |
1.97 | Like SiS2 consisting of edge sharing chains, melts at ~1700 K | 
|
| 2D silica[82] | hexagonal | Sheet-like bilayer structure | 
|
Safety[edit]
Inhaling finely divided crystalline silica can lead to severe inflammation of the lung tissue, silicosis, bronchitis, lung cancer, and systemic autoimmune diseases, such as lupus and rheumatoid arthritis. Inhalation of amorphous silicon dioxide, in high doses, leads to non-permanent short-term inflammation, where all effects heal.[83]
Other names[edit]
This extended list enumerates synonyms for silicon dioxide; all of these values are from a single source; values in the source were presented capitalized.[84]
- CAS 112945-52-5
- Acitcel
- Aerosil
- Amorphous silica dust
- Aquafil
- CAB-O-GRIP II
- CAB-O-SIL
- CAB-O-SPERSE
- Catalogue
- Colloidal silica[citation needed]
- Colloidal silicon dioxide
- Dicalite
- DRI-DIE Insecticide 67
- FLO-GARD
- Fossil flour
- Fumed silica
- Fumed silicon dioxide
- HI-SEL
- LO-VEL
- Ludox
- Nalcoag
- Nyacol
- Santocel
- Silica
- Silica aerogel
- Silica, amorphous
- Silicic anhydride
- Silikill
- Synthetic amorphous silica
- Vulkasil
See also[edit]
- Mesoporous silica
- Orthosilicic acid
- Silicon carbide
References[edit]
- ^ a b c d e Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1-4398-5511-0.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. «#0552». National Institute for Occupational Safety and Health (NIOSH).
- ^ a b NIOSH Pocket Guide to Chemical Hazards. «#0682». National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ Iler RK (1979). The Chemistry of Silica. New York: Wiley. ISBN 9780471024040.
- ^ a b Fernández LD, Lara E, Mitchell EA (2015). «Checklist, diversity and distribution of testate amoebae in Chile» (PDF). European Journal of Protistology. 51 (5): 409–24. doi:10.1016/j.ejop.2015.07.001. PMID 26340665. Archived (PDF) from the original on 2022-10-10.
- ^ a b Skinner BJ, Appleman DE (1963). «Melanophlogite, a cubic polymorph of silica» (PDF). Am. Mineral. 48: 854–867. Archived (PDF) from the original on 2022-10-10.
- ^ a b c d e f g Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- ^ Cuff YH (1996). Ceramic Technology for Potters and Sculptors. Philadelphia: University of Pennsylvania. pp. 93–95. ISBN 9780812213775.
- ^ De La Rocha C, Conley DJ (2017). «Mystical Crystals of Silica». Silica Stories. Cham: Springer. pp. 50–55. doi:10.1007/978-3-319-54054-2_4. ISBN 9783319540542.
- ^ a b c d e f g h Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 393–99. ISBN 978-0-08-022057-4.
- ^ Wells AF (1984). Structural Inorganic Chemistry. Oxford Science Publications. ISBN 9780198553700.
- ^ Kirfel A, Krane HG, Blaha P, et al. (2001). «Electron-density distribution in stishovite, SiO2: a new high-energy synchrotron-radiation study». Acta Crystallogr. A. 57 (6): 663–77. doi:10.1107/S0108767301010698. PMID 11679696.
- ^ Scherzer J (1978). «Dealuminated faujasite-type structures with SiO2/Al2O3 ratios over 100″. J. Catal. 54 (2): 285. doi:10.1016/0021-9517(78)90051-9.
- ^ Shell SM, Debenedetti PG, Panagiotopoulos AZ (2002). «Molecular structural order and anomalies in liquid silica» (PDF). Phys. Rev. E. 66 (1): 011202. arXiv:cond-mat/0203383. Bibcode:2002PhRvE..66a1202S. doi:10.1103/PhysRevE.66.011202. PMID 12241346. S2CID 6109212. Archived from the original (PDF) on 2016-06-04. Retrieved 2009-07-07.
- ^ Aksay IA, Pask JA, Davis RF (1979). «Densities of SiO2-Al2O3 Melts» (PDF). J. Am. Ceram. Soc. 62 (7–8): 332–336. doi:10.1111/j.1151-2916.1979.tb19071.x. Archived (PDF) from the original on 2022-10-10.
- ^ Jutzi P, Schubert U (2003). Silicon chemistry: from the atom to extended systems. Wiley-VCH. ISBN 9783527306473.
- ^ a b c d e Flörke OW, Graetsch HA, Brunk F, et al. (2018). «Silica». Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_583.pub3.
- ^ Berslien E (2012). An Introduction to Forensic Geoscience. Wiley & Sons. p. 138. ISBN 9781405160544.
- ^ Massey FP, Ennos AR, Hartley SE (2006). «Silica in grasses as a defence against insect herbivores: Contrasting effects on folivores and a phloem feeder». J. Anim. Ecol. 75 (2): 595–603. doi:10.1111/j.1365-2656.2006.01082.x. PMID 16638012.
- ^ Keeping MG, Kvedaras OL (2008). «Silicon as a plant defence against insect herbivory: Response to Massey, Ennos and Hartley». J. Anim. Ecol. 77 (3): 631–3. doi:10.1111/j.1365-2656.2008.01380.x. PMID 18341561.
- ^ Nevin, Charles Merrick (1925). Albany moulding sands of the Hudson Valley. University of the State of New York at Albany.
- ^ a b c d Greenhouse S (23 Aug 2013). «New Rules Would Cut Silica Dust Exposure». The New York Times. Retrieved 24 Aug 2013.
- ^ Ojovan MI (2004). «Glass formation in amorphous SiO2 as a percolation phase transition in a system of network defects». JETP Lett. 79 (12): 632–634. Bibcode:2004JETPL..79..632O. doi:10.1134/1.1790021. S2CID 124299526.
- ^ Elliott SR (1991). «Medium-range structural order in covalent amorphous solids». Nature. 354 (6353): 445–452. Bibcode:1991Natur.354..445E. doi:10.1038/354445a0. S2CID 4344891.
- ^ Atkins PW, Overton T, Rourke J, et al., eds. (2010). Shriver & Atkins’ inorganic chemistry (5th ed.). Oxford: Oxford University Press. p. 354. ISBN 9780199236176. OCLC 430678988.
- ^ a b «Cab-O-Sil Fumed Metal Oxides».
- ^ Cheraghian, Goshtasp; Wistuba, Michael P.; Kiani, Sajad; Barron, Andrew R.; Behnood, Ali (December 2021). «Rheological, physicochemical, and microstructural properties of asphalt binder modified by fumed silica nanoparticles». Scientific Reports. 11 (1): 11455. Bibcode:2021NatSR..1111455C. doi:10.1038/s41598-021-90620-w. PMC 8169902. PMID 34075083.
- ^ Barel AO, Paye M, Maibach HI (2014). Handbook of Cosmetic Science and Technology (4th ed.). CRC Press. p. 444. ISBN 9781842145654.
These soft-focus pigments, mainly composed of polymers, micas and talcs covered with rough or spherical particles of small diameters, such as silica or titanium dioxide, are used to optically reduce the appearance of wrinkles. These effects are obtained by optimizing outlines of wrinkles and reducing the difference of brightness due to diffuse reflection.
- ^ Barel AO, Paye M, Maibach HI (2014). Handbook of Cosmetic Science and Technology (4th ed.). CRC Press. p. 442. ISBN 9781842145654.
The silica is a multiporous ingredient, which absorbs the oil and sebum.
- ^ Bassett, Ross Knox (2007). To the Digital Age: Research Labs, Start-up Companies, and the Rise of MOS Technology. Johns Hopkins University Press. pp. 22–23. ISBN 9780801886393.
- ^ a b Lécuyer, Christophe; Brock, David C. (2010). Makers of the Microchip: A Documentary History of Fairchild Semiconductor. MIT Press. p. 111. ISBN 9780262294324.
- ^ a b Saxena, A (2009). Invention of integrated circuits: untold important facts. International series on advances in solid state electronics and technology. World Scientific. pp. 96–97. ISBN 9789812814456.
- ^ a b c «Martin Atalla in Inventors Hall of Fame, 2009». Retrieved 21 June 2013.
- ^ a b Black, Lachlan E. (2016). New Perspectives on Surface Passivation: Understanding the Si-Al2O3 Interface. Springer. p. 17. ISBN 9783319325217.
- ^ a b «Dawon Kahng». National Inventors Hall of Fame. Retrieved 27 June 2019.
- ^ Goodwin W, Linacre A, Hadi S (2007). An Introduction to Forensic Genetics. Wiley & Sons. p. 29. ISBN 9780470010259.
- ^ Calderone J (20 Aug 2015). «This cloud-like, futuristic material has been sneaking its way into your life since 1931». Business Insider. Retrieved 11 Feb 2019.
- ^ Thabet, Ahmed F.; Boraei, Hessien A.; Galal, Ola A.; El-Samahy, Magdy F. M.; Mousa, Kareem M.; Zhang, Yao Z.; Tuda, Midori; Helmy, Eman A.; Wen, Jian; Nozaki, Tsubasa (2021-07-14). «Silica nanoparticles as pesticide against insects of different feeding types and their non-target attraction of predators». Scientific Reports. 11 (1): 14484. Bibcode:2021NatSR..1114484T. doi:10.1038/s41598-021-93518-9. ISSN 2045-2322. PMC 8280210. PMID 34262071.
- ^ a b Meyer A, Sandler DP, Beane Freeman LE, et al. (2017). «Pesticide Exposure and Risk of Rheumatoid Arthritis among Licensed Male Pesticide Applicators in the Agricultural Health Study». Environmental Health Perspectives. 125 (7): 077010-1–077010-7. doi:10.1289/EHP1013. PMC 5744649. PMID 28718769.
- ^ Doering R, Nishi Y, eds. (2007). Handbook of Semiconductor Manufacturing Technology. CRC Press. ISBN 9781574446753.
- ^ Lee S (2006). Encyclopedia of chemical processing. CRC Press. ISBN 9780824755638.
- ^ Morgan DV, Board K (1991). An Introduction To Semiconductor Microtechnology (2nd ed.). Chichester, West Sussex, England: John Wiley & Sons. p. 72. ISBN 9780471924784.
- ^ Riordan M (2007). «The Silicon Dioxide Solution: How physicist Jean Hoerni built the bridge from the transistor to the integrated circuit». IEEE Spectrum. Retrieved 11 Feb 2019.
- ^ Chrystie, Robin S. M.; Ebertz, Felix L.; Dreier, Thomas; Schulz, Christof (2019-01-28). «Absolute SiO concentration imaging in low-pressure nanoparticle-synthesis flames via laser-induced fluorescence». Applied Physics B. 125 (2): 29. Bibcode:2019ApPhB.125…29C. doi:10.1007/s00340-019-7137-8. ISSN 1432-0649. S2CID 127735545.
- ^ Romero-Jaime, A. K.; Acosta-Enríquez, M. C.; Vargas-Hernández, D.; Tánori-Córdova, J. C.; Pineda León, H. A.; Castillo, S. J. (August 2021). «Synthesis and characterization of silica–lead sulfide core–shell nanospheres for applications in optoelectronic devices». Journal of Materials Science: Materials in Electronics. 32 (16): 21425–21431. doi:10.1007/s10854-021-06648-1. ISSN 0957-4522. S2CID 236182027.
- ^ Nandiyanto AB, Kim SG, Iskandar F, et al. (2009). «Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters». Microporous and Mesoporous Materials. 120 (3): 447–453. doi:10.1016/j.micromeso.2008.12.019.
- ^ Morgan DV, Board K (1991). An Introduction To Semiconductor Microtechnology (2nd ed.). Chichester, West Sussex, England: John Wiley & Sons. p. 27. ISBN 9780471924784.
- ^ Fleischer, Michael (1962). «New mineral names» (PDF). American Mineralogist. Mineralogical Society of America. 47 (2): 172–174. Archived (PDF) from the original on 2011-07-22.
- ^ Rodgers GE (2011). Descriptive Inorganic, Coordination, and Solid State Chemistry. Cengage Learning. pp. 421–2. ISBN 9781133172482.
- ^ Laine, Richard M.; Blohowiak, Kay Youngdahl; Robinson, Timothy R.; Hoppe, Martin L.; Nardi, Paola; Kampf, Jeffrey; Uhm, Jackie (17 October 1991). «Synthesis of pentacoordinate silicon complexes from SiO2» (PDF). Nature. 353 (6345): 642–644. Bibcode:1991Natur.353..642L. doi:10.1038/353642a0. hdl:2027.42/62810. S2CID 4310228. Archived (PDF) from the original on 2017-08-19.
- ^ Fournier RO, Rowe JJ (1977). «The solubility of amorphous silica in water at high temperatures and high pressures» (PDF). Am. Mineral. 62: 1052–1056. Archived (PDF) from the original on 2022-10-10.
- ^ Okamoto, Atsushi (2019). «Formation of silica particles from supercritical fluids and its impacts on the hydrological properties in the crust». EGU General Assembly Conference Abstracts: 4614. Bibcode:2019EGUGA..21.4614O.
- ^ Rondeau V, Jacqmin-Gadda H, Commenges D, et al. (2008). «Aluminum and Silica in Drinking Water and the Risk of Alzheimer’s Disease or Cognitive Decline: Findings from 15-Year Follow-up of the PAQUID Cohort». American Journal of Epidemiology. 169 (4): 489–96. doi:10.1093/aje/kwn348. PMC 2809081. PMID 19064650.
- ^ «Work Safely with Silica». CPWR — The Center for Construction Research and Training. Retrieved 11 Feb 2019.
- ^ «Action Plan for Lupus Research». National Institute of Arthritis and Musculoskeletal and Skin Diseases. National Institutes of Health. 2017. Retrieved 11 Feb 2019.
- ^ Reuzel PG, Bruijntjes JP, Feron VJ, et al. (1991). «Subchronic inhalation toxicity of amorphous silica and quartz dust in rats». Food Chem. Toxicol. 29 (5): 341–54. doi:10.1016/0278-6915(91)90205-L. PMID 1648030.
- ^ «Worker Exposure to Silica during Countertop Manufacturing, Finishing and Installation» (PDF). National Institute for Occupational Safety and Health and Occupational Safety and Health Administration. 2015. Archived (PDF) from the original on 2022-10-10. Retrieved 26 Feb 2015.
- ^ Hornung V, Bauernfeind F, Halle A, et al. (2008). «Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization». Nat. Immunol. 9 (8): 847–856. doi:10.1038/ni.1631. PMC 2834784. PMID 18604214.
- ^ Merchant JA, ed. (1986). Occupational Respiratory Diseases (PDF). Cincinnati, OH: US Department of Health and Human Services, NIOSH. doi:10.26616/NIOSHPUB86102. hdl:2027/uc1.31210023588922. DHHS (NIOSH) Publication Number 86-102.
- ^ NIOSH (2002) Hazard Review, Health Effects of Occupational Exposure to Respirable Crystalline Silica. Cincinnati, OH: U.S. Department of Health and Human Services, U.S. Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2002-129.
- ^ «Crystalline Factsheet» (PDF). Archived from the original (PDF) on 22 December 2017. Retrieved 3 August 2017.
- ^ «Silica, Crystalline». Retrieved 3 August 2017.
- ^ «Frequently Asked Questions». Retrieved 3 August 2017.
- ^ «If It’s Silica, It’s Not Just Dust!» (PDF). Archived (PDF) from the original on 2022-10-10. Retrieved 3 August 2017.
- ^ «What you should know about crystalline silica, silicosis, and Oregon OSHA silica rules» (PDF). Archived (PDF) from the original on 2022-10-10. Retrieved 3 August 2017.
- ^ Szymendera, Scott D. (January 16, 2018). Respirable Crystalline Silica in the Workplace: New Occupational Safety and Health Administration (OSHA) Standards (PDF). Washington, DC: Congressional Research Service. Archived (PDF) from the original on 2022-10-10. Retrieved 27 January 2018.
- ^ Lager G. A.; Jorgensen J. D.; Rotella F.J. (1982). «Crystal structure and thermal expansion of a-quartz SiO2 at low temperature». Journal of Applied Physics. 53 (10): 6751–6756. Bibcode:1982JAP….53.6751L. doi:10.1063/1.330062.
- ^ Wright, A. F.; Lehmann, M. S. (1981). «The structure of quartz at 25 and 590 °C determined by neutron diffraction». Journal of Solid State Chemistry. 36 (3): 371–80. Bibcode:1981JSSCh..36..371W. doi:10.1016/0022-4596(81)90449-7.
- ^ a b Kihara, Kuniaki; Matsumoto, Takeo; Imamura, Moritaka (1986). «Structural change of orthorhombic-Itridymite with temperature: A study based on second-order thermal-vibrational parameters». Zeitschrift für Kristallographie. 177 (1–2): 27–38. Bibcode:1986ZK….177…27K. doi:10.1524/zkri.1986.177.1-2.27.
- ^ Downs R. T.; Palmer D. C. (1994). «The pressure behavior of a cristobalite» (PDF). American Mineralogist. 79: 9–14. Archived (PDF) from the original on 2022-10-10.
- ^ Wright, A. F.; Leadbetter, A. J. (1975). «The structures of the β-cristobalite phases of SiO2 and AlPO4«. Philosophical Magazine. 31 (6): 1391–401. Bibcode:1975PMag…31.1391W. doi:10.1080/00318087508228690.
- ^ Shropshire, Joseph; Keat, Paul P.; Vaughan, Philip A. (1959). «The crystal structure of keatite, a new form of silica». Zeitschrift für Kristallographie. 112 (1–6): 409–13. Bibcode:1959ZK….112..409S. doi:10.1524/zkri.1959.112.1-6.409.
- ^ Miehe, Gerhard; Graetsch, Heribert (1992). «Crystal structure of moganite: a new structure type for silica». European Journal of Mineralogy. 4 (4): 693–706. Bibcode:1992EJMin…4..693M. doi:10.1127/ejm/4/4/0693.
- ^ Levien L.; Prewitt C. T. (1981). «High-pressure crystal structure and compressibility of coesite» (PDF). American Mineralogist. 66: 324–333. Archived (PDF) from the original on 2022-10-10.
- ^ Smyth J. R.; Swope R. J.; Pawley A. R. (1995). «H in rutile-type compounds: II. Crystal chemistry of Al substitution in H-bearing stishovite» (PDF). American Mineralogist. 80 (5–6): 454–456. Bibcode:1995AmMin..80..454S. doi:10.2138/am-1995-5-605. S2CID 196903109. Archived (PDF) from the original on 2022-10-10.
- ^ Dera P.; Prewitt C. T.; Boctor N. Z.; Hemley R. J. (2002). «Characterization of a high-pressure phase of silica from the Martian meteorite Shergotty». American Mineralogist. 87 (7): 1018. Bibcode:2002AmMin..87.1018D. doi:10.2138/am-2002-0728. S2CID 129400258.
- ^ Seifertite. Mindat.org.
- ^ Nakagawa T.; Kihara K.; Harada K. (2001). «The crystal structure of low melanophlogite». American Mineralogist. 86 (11–12): 1506. Bibcode:2001AmMin..86.1506N. doi:10.2138/am-2001-11-1219. S2CID 53525827.
- ^ Rosemarie Szostak (1998). Molecular sieves: Principles of Synthesis and Identification. Springer. ISBN 978-0-7514-0480-7.
- ^ Weiss, Alarich; Weiss, Armin (1954). «Über Siliciumchalkogenide. VI. Zur Kenntnis der faserigen Siliciumdioxyd-Modifikation». Zeitschrift für Anorganische und Allgemeine Chemie. 276 (1–2): 95–112. doi:10.1002/zaac.19542760110.
- ^ Björkman, T; Kurasch, S; Lehtinen, O; Kotakoski, J; Yazyev, O. V.; Srivastava, A; Skakalova, V; Smet, J. H.; Kaiser, U; Krasheninnikov, A. V. (2013). «Defects in bilayer silica and graphene: common trends in diverse hexagonal two-dimensional systems». Scientific Reports. 3: 3482. Bibcode:2013NatSR…3E3482B. doi:10.1038/srep03482. PMC 3863822. PMID 24336488.
- ^ Johnston CJ, Driscoll KE, Finkelstein JN, et al. (2000). «Pulmonary Chemokine and Mutagenic Responses in Rats after Subchronic Inhalation of Amorphous and Crystalline Silica». Toxicological Sciences. 56 (2): 405–413. doi:10.1093/toxsci/56.2.405. PMID 10911000.
- ^ Lewis, Grace Ross (1999). 1001 chemicals in everyday products (2nd ed.). John Wiley & Sons (Wiley-Interscience). pp. 250–1. ISBN 0-471-29212-5 – via Internet Archive.
External links[edit]
- Chisholm, Hugh, ed. (1911). «Silica» . Encyclopædia Britannica (11th ed.). Cambridge University Press.
- Tridymite, International Chemical Safety Card 0807
- Quartz, International Chemical Safety Card 0808
- Cristobalite, International Chemical Safety Card 0809
- Amorphous, NIOSH Pocket Guide to Chemical Hazards
- Crystalline, as respirable dust, NIOSH Pocket Guide to Chemical Hazards
- Formation of silicon oxide layers in the semiconductor industry. LPCVD and PECVD method in comparison. Stress prevention.
- Quartz (SiO2) piezoelectric properties
- Silica (SiO2) and water
- Epidemiological evidence on the carcinogenicity of silica: factors in scientific judgement by C. Soutar and others. Institute of Occupational Medicine Research Report TM/97/09
- Scientific opinion on the health effects of airborne silica by A Pilkington and others. Institute of Occupational Medicine Research Report TM/95/08
- The toxic effects of silica Archived 2016-04-15 at the Wayback Machine by A. Seaton and others. Institute of Occupational Medicine Research Report TM/87/13
- Structure of precipitated silica
диоксид кремния
-
1
диоксид кремния
- silicon dioxide
Русско-английский словарь нормативно-технической терминологии > диоксид кремния
-
2
диоксид кремния
Русско-английский новый политехнический словарь > диоксид кремния
-
3
диоксид кремния
Русско-английский словарь по информационным технологиям > диоксид кремния
-
4
диоксид кремния
Универсальный русско-английский словарь > диоксид кремния
-
5
диоксид кремния
Русско-английский словарь по нефти и газу > диоксид кремния
-
6
диоксид кремния
Русско-английский словарь по электронике > диоксид кремния
-
7
диоксид кремния
Русско-английский словарь по радиоэлектронике > диоксид кремния
-
8
диоксид кремния
Русско-английский словарь по строительству и новым строительным технологиям > диоксид кремния
-
9
диоксид кремния
Русско-английский словарь по микроэлектронике > диоксид кремния
-
10
диоксид кремния
silicon dioxide, silicon oxide
Русско-английский политехнический словарь > диоксид кремния
-
11
диоксид кремния с имплантированными ионами бора
Универсальный русско-английский словарь > диоксид кремния с имплантированными ионами бора
-
12
диоксид кремния, легированный фосфором
Универсальный русско-английский словарь > диоксид кремния, легированный фосфором
-
13
диоксид кремния, содержащий воду
Универсальный русско-английский словарь > диоксид кремния, содержащий воду
-
14
диоксид кремния, сформированный в канавках
Универсальный русско-английский словарь > диоксид кремния, сформированный в канавках
-
15
диоксид кремния с имплантированными ионами бора
Русско-английский словарь по микроэлектронике > диоксид кремния с имплантированными ионами бора
-
16
Осаждённый диоксид кремния
Универсальный русско-английский словарь > Осаждённый диоксид кремния
-
17
активный диоксид кремния
Универсальный русско-английский словарь > активный диоксид кремния
-
18
аморфный диоксид кремния
Универсальный русско-английский словарь > аморфный диоксид кремния
-
19
высокодисперсный диоксид кремния
Универсальный русско-английский словарь > высокодисперсный диоксид кремния
-
20
гетеропереход кремний-диоксид кремния
Универсальный русско-английский словарь > гетеропереход кремний-диоксид кремния
Страницы
- Следующая →
- 1
- 2
- 3
См. также в других словарях:
-
ДИОКСИД КРЕМНИЯ — ДИОКСИД КРЕМНИЯ, см. КРЕМНЕЗЕМ … Научно-технический энциклопедический словарь
-
диоксид кремния — — [А.С.Гольдберг. Англо русский энергетический словарь. 2006 г.] Тематики энергетика в целом EN silicon dioxide … Справочник технического переводчика
-
Диоксид кремния — (Si02) кремнезем, основная составляющая кварцевого песка сырьевого компонента обычных и силикатных бетонов. Существует в кристаллической, практически инертной и аморфной, реакционноспособной формах. [Ушеров Маршак А. В. Бетоноведение: лексикон. М … Энциклопедия терминов, определений и пояснений строительных материалов
-
Диоксид кремния — Кварц Кварцевое стекло Диоксид кремния (оксид кремния (IV), кремнезём, SiO2) бесцветные кристаллы, tпл 1713 1728 °C, обладают высокой твёрдостью и прочностью … Википедия
-
Диоксид кремния — кремниевый ангидрид, кремнезем, SiO2 соединение Si с O2. В форме минерала кварца, разновидностей кремнезема и силикатов Диоксид кремния составляет около 12% массы земной коры. Широко применяют в литейном производстве в качестве футеровки и кладки … Энциклопедический словарь по металлургии
-
ДИОКСИД КРЕМНИЯ — кремниевый ангидрид, кремнезем, SiO2 соединение Si с О2. В форме минерала кварца, разновидностей кремнезема и силикатов диоксид кремния составляет около 12% массы земной коры. Широко применяют в литейном производстве в качестве футеровки и кладки … Металлургический словарь
-
диоксид кремния — silicio dioksidas statusas T sritis radioelektronika atitikmenys: angl. silica; silicon dioxide; silox vok. Siliziumdioxid, n rus. диоксид кремния, m pranc. dioxyde de silicium, m … Radioelektronikos terminų žodynas
-
диоксид кремния, легированный фосфором — fosforu legiruotas silicio dioksidas statusas T sritis radioelektronika atitikmenys: angl. phosphorous doped silicon dioxide vok. phosphordotiertes Siliziumdioxid, n rus. диоксид кремния, легированный фосфором, m pranc. dioxyde de silicium dopé… … Radioelektronikos terminų žodynas
-
диоксид кремния в канавках — griovelių silicio dioksidas statusas T sritis radioelektronika atitikmenys: angl. recessed silicon dioxide vok. in den Isolationsgräben erzeugtes Siliziumdioxid, n rus. диоксид кремния в канавках, m pranc. dioxyde de silicium formé en rainures, m … Radioelektronikos terminų žodynas
-
Химически активный диоксид кремния — (SiO2) – та фракция диоксида кремния, которая растворима после обработки соляной кислотой (НСl) и при кипении раствора гидроксида калия (КОН). Примечание: Количество химически активного диоксида кремния определяется путем вычитания из… … Энциклопедия терминов, определений и пояснений строительных материалов
-
естественный диоксид кремния — gamtinis silicio dioksidas statusas T sritis radioelektronika atitikmenys: angl. native grown silicon dioxide; natural silicon dioxide vok. Eigensiliziumdioxid, n rus. естественный диоксид кремния, m pranc. dioxyde de silicium de source, m … Radioelektronikos terminų žodynas
Содержание
- Свойства
- Полиморфизм
- Химические свойства
- Получение
- Применение
- Пористые кремнезёмы
- Токсичность
Диоксид кремния — оксид кремния. Бесцветные кристаллы с температурой плавления +1713…+1728 °C, обладающие высокой твёрдостью и прочностью.
| Диоксид кремния | |
|---|---|
| Общие | |
| Систематическое наименование |
оксид кремния (IV) |
| Традиционные названия | диоксид кремния; кремнезём |
| Хим. формула | SiO2 |
| Физические свойства | |
| Молярная масса | 60.0843 г/моль |
| Плотность | от 1,96 до 2,6 г/см³ |
| Удельное электрическое сопротивление | от 10¹¹ до 10¹³ Ом·м |
| Термические свойства | |
| Температура | |
| • плавления | 1600 °C |
| • кипения | 2950 °C |
| Давление пара | 0 ± 1 мм рт.ст. |
| Классификация | |
| Рег. номер CAS | 7631-86-9 |
| PubChem | 24261 |
| Рег. номер EINECS | 231-545-4 |
| SMILES |
O=[Si]=O |
| InChI |
1S/O2Si/c1-3-2 VYPSYNLAJGMNEJ-UHFFFAOYSA-N |
| Кодекс Алиментариус | E551 |
| RTECS | VV7565000 |
| ChEBI | 30563 |
| ChemSpider | 22683 |
| Безопасность | |
| Предельная концентрация | 3 мг/м³ |
| ЛД50 | 3500 мг/кг |
| Токсичность | низкая |
| Пиктограммы ECB |  |
| NFPA 704 |
Диоксид кремния — главный компонент почти всех земных горных пород, в частности, кизельгура. Из кремнезёма и силикатов состоит 87 % массы литосферы. В крови и плазме человека концентрация кремнезёма составляет 0,001 % по массе.
Свойства
- Относится к группе кислотных оксидов.
- При нагревании взаимодействует с основными оксидами и щелочами.
- Молярная масса: 60,084 г/моль
- Реагирует с плавиковой кислотой.
- SiO2 относится к группе стеклообразующих оксидов, то есть склонен к образованию переохлаждённого расплава — стекла.
- Диэлектрик (электрический ток не проводит, если не имеет примесей и не нагревается).
Полиморфизм
Диоксид кремния имеет несколько полиморфных модификаций.
Самая распространённая из них на поверхности земли — α-кварц — кристаллизуется в тригональной сингонии. При нормальных условиях диоксид кремния чаще всего находится в полиморфной модификации α-кварца, которая при температуре выше +573 °C обратимо переходит в β-кварц. При дальнейшем повышении температуры кварц переходит в тридимит и кристобалит. Эти полиморфные модификации устойчивы при высоких температурах и низких давлениях.
В природе также встречаются формы — опал, халцедон, кварцин, лютецит, аутигенный кварц, которые относятся к группе кремнезёма. Опал (SiO2·nH2O) в шлифе бесцветен, изотропен, имеет отрицательный рельеф, отлагается в морских водоёмах, входит в состав многих кремнистых пород. Халцедон, кварцин, лютецит — SiO2 — представляют собой скрытокристаллические разновидности кварца. Образуют волокнистые агрегаты, розетки, сферолиты, бесцветные, голубоватые, желтоватые. Отличаются между собой некоторыми свойствами — у халцедона и кварцина — прямое погасание, у лютецита — косое, у халцедона — отрицательное удлинение.
При высоких температуре и давлении диоксид кремния сначала превращается в коэсит (который в 1953 году был синтезирован американским химиком Лорингом Коэсом), а затем — в стишовит (который в 1961 году был синтезирован С. М. Стишовым, а в 1962 году был обнаружен в кратере Бэрринджера (кратере Аризонского метеорита). Согласно некоторым исследованиям[каким?], стишовит слагает значительную часть мантии, так что вопрос о том, какая разновидность SiO2 наиболее распространена на Земле, пока не имеет однозначного ответа.
Также имеет аморфную модификацию — кварцевое стекло.
Химические свойства
Диоксид кремния SiO2 — кислотный оксид, не реагирующий с водой.
Химически стоек к действию кислот, но реагирует с газообразным фтороводородом:
и плавиковой кислотой:
Эти две реакции широко используют для плавления стекла.
При сплавлении SiO2 с щелочами и основными оксидами, а также с карбонатами активных металлов образуются силикаты — соли не имеющих постоянного состава очень слабых, нерастворимых в воде кремниевых кислот общей формулы xH2O·ySiO2 (довольно часто в литературе упоминаются не кремниевые кислоты, а кремниевая кислота, хотя фактически речь при этом идёт об одном и том же веществе).
Например, может быть получен ортосиликат натрия:
метасиликат кальция:
или смешанный силикат кальция и натрия:
Из силиката Na2CaSi6O14 (Na2O·CaO·6SiO2) изготовляют оконное стекло.
Большинство силикатов не имеет постоянного состава. Из всех силикатов растворимы в воде только силикаты натрия и калия. Растворы этих силикатов в воде называют жидким стеклом. Из-за гидролиза эти растворы характеризуются сильно щелочной средой. Для гидролизованных силикатов характерно образование не истинных, а коллоидных растворов. При подкислении растворов силикатов натрия или калия выпадает студенистый белый осадок гидратированных кремниевых кислот.
Главным структурным элементом как твёрдого диоксида кремния, так и всех силикатов, выступает группа [SiO4/2], в которой атом кремния Si окружен тетраэдром из четырёх атомов кислорода О. При этом каждый атом кислорода соединён с двумя атомами кремния. Фрагменты [SiO4/2] могут быть связаны между собой по-разному. Среди силикатов по характеру связи в них фрагментов [SiO4/2] выделяют островные, цепочечные, ленточные, слоистые, каркасные и другие.
Получение
Синтетический диоксид кремния получают нагреванием кремния до температуры +400…+500 °C в атмосфере кислорода, при этом кремний окисляется до диоксида SiO2. А также термическим оксидированием при больших температурах.
В лабораторных условиях синтетический диоксид кремния может быть получен действием кислот, даже слабой уксусной, на растворимые силикаты. Например:
кремниевая кислота сразу распадается на воду и SiO2, выпадающий в осадок.
Натуральный диоксид кремния в виде песка используется там, где не требуется высокая чистота материала.
Применение
Аморфный непористый диоксид кремния применяется в пищевой промышленности в качестве вспомогательного вещества E551, препятствующего слёживанию и комкованию, в парафармацевтике (зубные пасты), в фармацевтической промышленности в качестве вспомогательного вещества (внесён в большинство фармакопей), для стабилизации суспензий и линиментов, в качестве загустителя мазевых основ, наполнителя таблеток и суппозиториев. Он входит в состав композиции пломбировочных материалов, снижает гигроскопичность сухих экстрактов, замедляет выход БАВ из различных лекарственных форм; в качестве пищевых добавок и сорбента, а также матриц для создания лекарственных форм с заданными свойствами — так как нет кристаллической структуры (аморфен) — безопасен, а также в качестве пищевой добавки или лекарственного препарата в качестве энтеросорбента Полисорб МП с широким спектром применения с учётом высокой удельной поверхности сорбции (в интервале 300—400 м²) на 1 г основного вещества.
Диоксид кремния применяют в производстве стекла, керамики, абразивов, бетонных изделий, для получения кремния, как наполнитель в производстве резин, при производстве кремнезёмистых огнеупоров, в хроматографии и другом.
Кристаллы кварца обладают пьезоэлектрическими свойствами и поэтому используются в радиотехнике, ультразвуковых установках, в зажигалках.
Искусственно полученные плёнки диоксида кремния используются в качестве изолятора при производстве микросхем и других электронных компонентов.
Также используется для производства волоконно-оптических кабелей. Используется чистый плавленый диоксид кремния с добавкой в него некоторых специальных ингредиентов.
Кремнезёмная нить также используется в нагревательных элементах электронных сигарет, так как хорошо впитывает жидкость и не разрушается под нагревом спирали.
Также диоксид кремния нашёл наиболее широкое применение в шинной промышленности, производстве РТИ и пластмасс, химической промышленности, машиностроении, а в ряде конкретных операций:
- как носитель катализаторов и химических средств защиты растений;
- в качестве сорбентов и фильтровальных порошков для регенерации нефтепродуктов;
- как высококачественный флюс в процессах цветной металлургии;
- как сырьё для производства экологически чистого стекла, стеклотары и хрусталя;
- как наполнитель в бумагу и картон для получения гигиенически чистых упаковочных материалов для пищевой промышленности;
- фильтрующие порошки для пива, масел, соков, матирующие добавки в лаки и краски;
- для получения карбида кремния в машиностроении — керамические двигатели, детали для авиастроительного комплекса;
- для получения кристаллического кремния в электронной и электротехнической промышленностях, керамические электроизоляторы, стекловолокна, волоконная оптика, супертонкое волокно;
- для синтеза искусственных цеолитов в нефтехимии — крекинг нефти и прочее.
Крупные прозрачные кристаллы кварца используются в качестве полудрагоценных камней; бесцветные кристаллы называют горным хрусталём, фиолетовые — аметистами, жёлтые — цитрином.
В микроэлектронике диоксид кремния является одним из основных материалов. Его применяют в качестве изолирующего слоя, а также в качестве защитного покрытия. Получают в виде тонких плёнок термическим окислением кремния, химическим осаждением из газовой фазы, магнетронным распылением.
Пористые кремнезёмы
Пористые кремнезёмы получают различными методами.
Силохром получают путём агрегирования аэросила, который, в свою очередь, получают сжиганием силана (SiH4). Силохром характеризуется высокой чистотой, низкой механической прочностью. Характерный размер удельной поверхности 60—120 м²/г. Применяется в качестве сорбента в хроматографии, наполнителя резин, катализе.
Силикагель получают путём высушивания геля кремниевой кислоты. В сравнении с силохромом обладает меньшей чистотой, однако может обладать чрезвычайно развитой поверхностью: обычно от 300 м²/г до 700 м²/г .
Кремниевый аэрогель приблизительно на 99,8 % состоит из воздуха и может иметь плотность до 1,9 кг/м³ (всего в 1,5 раза больше плотности воздуха).
Токсичность
- Вещество малотоксично. ПДК в рабочей зоне — 3 мг/м³. ЛД50 на крысах — 3500 мг/кг.
- При попадании диоксида кремния в ткани организма происходит возникновение и постепенное развитие гранулом. При вдыхании пыли происходит раздражение дыхательных путей, также возникают различные заболевания пищевого тракта. Постоянное воздействие пыли может вызвать силикоз лёгких.
Химическое название
Оксид кремния (IV)
Химические свойства
Диоксид кремния, что это такое? Согласно Википедии, четырехвалентный оксид кремния входит в состав практически всех горных пород. Это химическое соединение имеет вид бесцветных кристаллов, с достаточно высокой температурой плавления. Формула Диоксида Кремния: SiO2. Химическая формула кремнезема совпадает с формулой Диоксида Кремния. Температура плавления – около 1600 градусов Цельсия.
Вещество относят к группе кислотных оксидов, является диэлектриком, и имеет несколько полиморфных модификаций кристаллов. Под действием высоких температур и давления вещество превращается в коэсит и стишовит, имеет различные модификации и формы, кварцин, опал, аутигенный кварц, халцедон; аморфный Диоксид Кремния – это кварцевое стекло.
Применение кремнезема
Вещество вследствие разнообразия форм, применяется в различных областях. Минерал используют при производстве стекла, абразивов, изделий из бетона и керамики; в качестве наполнителя во время производства резины, для получения кремния; при производстве огнеупорных материалов; в хроматографии. Кварцевые кристаллы применяют для производства зажигалок, ультразвуковых установок, в радиотехнике. Некоторые водоросли способствуют накоплению кремнезема в биосфере и выполняют биохимическую функцию. Соединение также применяют в качестве эмульгатора в пищевой промышленности (Е551), добавляют в состав зубной пасты. Применяют в виде изолятора, при производстве волоконно-оптических кабелей, используют в виде нагревательного элемента в электронных сигаретах; в ювелирном деле и так далее. Распространено применение Диоксида Кремния в медицине в качестве вспомогательного вещества, пищевой добавки или в виде энтеросорбента.
Диоксид кремния: вред и польза
Вещество нанести особого вреда организму не может, так как при проникновении в желудочно-кишечный тракт не всасывается через стенки желудка и выводится в неизмененном виде. Пищевая добавка Е 551 присутствует во многих продуктах питания, в сахаре, сухом молоке и славках, чипсах, сухариках, алкогольных напитках и кондитерских изделиях. При правильном использовании лекарственных препаратов вред кремния диоксида коллоидного также отсутствует.
Фармакологическое действие
Адсорбирующее, регенерирующее.
Фармакодинамика и фармакокинетика
Кремнезем имеет достаточно высокую абсорбционную способность. Вещество связывает и выводит из организма различные ферменты, антитела, ангигены, токсины, продукты тканевого распада, микроорганизмы и пищевые аллергены. Вещество активно применяют для эвакуации некоторых лекарств, воды и ядов. Средство после проникновения в пищеварительный тракт не подвергается системной абсорбции, не накапливается в организме.
При местном использовании вещество препятствует некротическим изменениям тканей, способствует заживлению ран.
Показания к применению
В медицине используют Кремния Диоксид коллоидный:
- при кишечных инфекциях, пищевых токсикоинфекциях, аллергии;
- при экзогенной и эндогенной интоксикации;
- в рамках комплексного лечения острых отравлений;
- при алкогольной абстиненции;
- при лечении гнойно-воспалительных заболеваний мягких тканей, абсцессов, гнойных ран, флегмоны, мастита.
Противопоказания
Вещество противопоказано для системного использования при язве желудка и 12-перстной кишки во время обострения; при эрозии желудка и непроходимости кишечника. Лекарство не наносят на гранулирующие и чистые асептические раны.
Побочные действия
Кремнезем при приеме внутрь может вызвать несварение желудка, запор. При местном воздействии – образовать корку, препятствующую нормальной аэрации поверхности раны.
Инструкция по применению (Способ и дозировка)
Вещество принимают внутрь в соответствии с инструкцией, которая прилагается к препарату.
Средство используют наружно по рекомендации врача.
Передозировка
При передозировке у пациентов может возникнуть запор и несварение желудка. Нет сведений о случаях передозировки веществом.
Взаимодействие
Лекарство при пероральном приеме обладает способностью снижать эффективность одновременно принимаемых внутрь лекарств. Следует соблюдать промежуток в один час между приемами прочих лекарств.
При сочетании препаратов Диоксида Кремния с ацетилсалициловой кислотой усиливаются процессы дезагрегации тромбоцитов.
При одновременном приеме лекарства, никотиновой кислоты и атоксила повышается уровень холестерина ЛПВП.
Средство при проведении интракорпоральной сорбционной детоксикации рекомендуется сочетать с бифураном, фурацилином, хлоргексидин биглюконатом.
Условия продажи
Безрецептурный отпуск.
При беременности и лактации
Лекарство можно назначать во время кормления грудью и при беременности.
Препараты, в которых содержится (Аналоги)
Аэросил, Полисорб МП, Атоксил, Силикс, Максисорб.
Отзывы
Отзывы о препаратах на основе данного средства:
- “… Использую это лекарство давно. Очень мне помогает при аллергии и пищевых отравлениях”;
- “… Сначала принимала, чтобы очистить организм, сбросить лишний вес. Теперь это лекарство всегда в моей аптечке”;
- “… Муж недавно отравился сильно на работе. Начали пить этот препарат и уже через сутки все симптомы прошли”;
- “… Второй день пью его от крапивницы. Уже сошли пятна, и ушел зуд. Решила, что пропью его до конца курса. Хорошее лекарство”.
Цена, где купить
Купить Кремния Диоксид в виде порошка для приготовления суспензии Полисорб МП можно за 40 рублей, за пакет, весом 3 грамма.
Оксид кремния

4.5
Средняя оценка: 4.5
Всего получено оценок: 101.
4.5
Средняя оценка: 4.5
Всего получено оценок: 101.
Кремний проявляет переменную валентность (II, IV), поэтому может образовывать два оксида кремния – монооксид и диоксид. Они отличаются физическими и химическими свойствами. Подробнее об оксидах кремния говорим в этой статье.
Монооксид
Формула оксид кремния (II) – SiO. Это вязкое, похожее на смолу вещество. Сохраняет аморфное состояние и не окисляется при обычных условиях. Не образует соли, не проводит электричество.
Монооксид получают двумя способами:
- нагреванием (температура выше 400°C) кремния при недостатке кислорода:
2Si + O2 → 2SiO;
- методом Чохральского при восстановлении диоксида при высоких температурах:
SiO2 + Si → 2SiO.
В твёрдом состоянии монооксид представляет собой тёмно-коричневый порошок. Проявляет прочность и инертность в реакциях с кислотами. Растворим в плавиковой кислоте.
Химические свойства монооксида кремния:
- разлагается при нагревании:
2SiO → 2Si + O2;
- реагирует с парами воды при нагревании до 500°C:
SiO + H2O → SiO2 + H2;
- взаимодействует с углекислым газом при нагревании:
SiO + СО2 → SiO2 + CO;
- реагирует с хлором при температуре 800°C, образуя жидкий хлорид кремния:
2SiO + 4Cl2 → 2SiCl4 + O2.
Монооксид не встречается на Земле в естественной среде. Однако газообразный монооксид входит в состав межзвёздных пылевых облаков.
Диоксид
Оксид кремния (IV) – SiO2. Это твёрдое кристаллическое тугоплавкое вещество, нерастворимое в воде. Не проводит электрический ток.
Формулу диоксида имеют песок, кварц, горный хрусталь, яшма, агат, аметист и другие горные породы. Диоксид входит в состав 87 % литосферы.
Диоксид кремния имеет немолекулярное строение. Кристаллическая решётка состоит из атомов кремния и кислорода, связанных ковалентными связями. К каждому атому кремния присоединено четыре атома кислорода, а каждый атом кислорода связан с двумя атомами кремния.
Диоксид можно получить в лаборатории, нагрев кремний до 400-500°C в присутствии кислорода:
Si + O2 → SiO2.
Диоксид образуется при действии кислот на растворимые силикаты. В результате образующаяся кремниевая кислота распадается на воду и диоксид:
- Na2SiO3 + 2CH3COOH → 2CH3COONa + H2SiO3↓;
- H2SiO3 → H2O + SiO2.
При нормальных условиях реагирует только с плавиковой кислотой. Со щелочами и основными оксидами реагирует при нагревании. Не реагирует с водой. Основные свойства с химическими уравнениями представлены в таблице.
|
Взаимодействие |
Описание |
Уравнение |
|
С неметаллами |
Реагирует с водородом и углеродом с образованием кремния и карборунда соответственно |
– SiO2 + 2Н2 → Si + 2H2O; – SiO2 + 3С → SiC + 2CO |
|
С активными металлами |
Реагирует при температуре выше 1000°С с образованием кремния. При избытке металла образуются силициды |
– SiO2 + 2Mg → Si + 2MgO; – SiO2 + 4Mg → Mg2Si + 2MgO |
|
С фтороводородом |
Реагирует с газом и плавиковой кислотой при нормальных условиях |
– SiO2 + 4HF → SiF4 + 2H2O; – SiO2 + 6HF → H2[SiF6] + 2H2O |
|
Со щелочами |
Сплавляется с образованием силикатов |
SiO2 + 4NaOH → Na4SiO4 + 2H2O |
|
С оксидами |
Реагирует при высоких температурах |
SiO2 + MgO → MgSiO3 |
|
С карбонатами щелочных металлов |
Взаимодействует при нагревании |
SiO2 + K2CO3 → K2SiO3 + CO2 |
Диоксид кремния используется для производства стекла, силикагеля, бетона.
Что мы узнали?
Кремний образует два оксида – монооксид и диоксид. Монооксид – аморфное вещество, не образующее соли. Взаимодействует при нагревании с водяным паром, углекислым газом, хлором. Разлагается на простые вещества при нагревании. Диоксид – песок и его производные. Это кристаллическое вещество с немолекулярным строением. Не реагирует с водой, кислотами (исключение – плавиковая кислота). Взаимодействует с неметаллами, металлами, фтороводородом, щелочами, оксидами, карбонатами. Оксиды кремния – диэлектрики.
Тест по теме
Доска почёта

Чтобы попасть сюда — пройдите тест.
-
Александр Котков
5/5
Оценка доклада
4.5
Средняя оценка: 4.5
Всего получено оценок: 101.
А какая ваша оценка?
Оксид кремния (IV)
Физические свойства и нахождение в природе
Оксид кремния (IV) SiO2 – это твердое вещество с атомной кристаллической решеткой. В природе встречается в виде кварца, речного песка, кремнезема и прочих модификаций:
Химические свойства
Оксид кремния (IV) – типичный кислотный оксид. За счет кремния со степенью окисления +4 проявляет слабые окислительные свойства.
1. Как кислотный оксид, диоксид кремния (IV) взаимодействует с растворами и расплавами щелочей и в расплаве с основными оксидами. При этом образуются силикаты.
Например, диоксид кремния взаимодействует с гидроксидом калия:
SiO2 + 2KOH → K2SiO3 + H2O
Еще пример: диоксид кремния взаимодействует с оксидом кальция.
SiO2 + CaO → CaSiO3
2. Оксид кремния (IV) не взаимодействует с водой, т.к. кремниевая кислота нерастворима.
3. Оксид кремния (IV) реагирует при сплавлении с карбонатами щелочных металлов. При этом работает правило: менее летучий оксид вытесняет более летучий оксид из солей при сплавлении.
Например, оксид кремния (IV) взаимодействует с карбонатом калия. При этом образуется силикат калия и углекислый газ:
SiO2 + K2CO3 → K2SiO3 + CO2
4. Из кислот диоксид кремния реагирует только с плавиковой или с газообразным фтороводородом:
SiO2 + 6HF(г) = SiF4 + H2O
SiO2 + 6HF(р-р) → H2[SiF6] + 2H2O
5. При температуре выше 1000 °С оксид кремния реагирует с активными металлами, при этом образуется кремний.
Например, оксид кремния взаимодействует с магнием с образованием кремния и оксида магния:
SiO2 + 2Mg → Si + 2MgO
Видеоопыт взаимодействия оксида кремния (IV) с магнием можно посмотреть здесь.
При избытке восстановителя образуются силициды:
SiO2 + 4Mg → Mg2Si + 2MgO
6. Оксид кремния (IV) взаимодействует с неметаллами.
Например, оксид кремния (IV) реагирует с водородом в жестких условиях. При этом оксид кремния проявляет окислительные свойства:
SiO2 + 2Н2 → Si + 2Н2O
Еще пример: оксид кремния взаимодействует с углеродом. При этом образуется карборунд и угарный газ:
SiO2 + 3С → SiС + 2СО
При сплавлении оксид кремния взаимодействует с фосфатом кальция и углем:
3SiO2 + Ca3(PO4)2 + 5C → 3CaSiO3 + 5CO + 2P