From Wikipedia, the free encyclopedia

Aluminium trichloride hexahydrate, pure (top), and contaminated with iron(III) chloride (bottom) |
||
|
||
| Names | ||
|---|---|---|
| IUPAC name
Aluminium chloride |
||
| Other names
Aluminium(III) chloride |
||
| Identifiers | ||
|
CAS Number |
|
|
|
3D model (JSmol) |
|
|
| ChEBI |
|
|
| ChemSpider |
|
|
| ECHA InfoCard | 100.028.371 |
|
| EC Number |
|
|
|
Gmelin Reference |
1876 | |
|
PubChem CID |
|
|
| RTECS number |
|
|
| UNII |
|
|
|
CompTox Dashboard (EPA) |
|
|
|
InChI
|
||
|
SMILES
|
||
| Properties | ||
|
Chemical formula |
AlCl3 | |
| Molar mass |
[1] |
|
| Appearance | Colourless crystals, hygroscopic | |
| Density |
[1] |
|
| Melting point |
|
|
|
Solubility in water |
|
|
| Solubility |
|
|
| Vapor pressure |
[2] |
|
| Viscosity |
[2] |
|
| Structure | ||
|
Crystal structure |
Monoclinic, mS16 | |
|
Space group |
C12/m1, No. 12[3] | |
|
Lattice constant |
a = 0.591 nm, b = 0.591 nm, c = 1.752 nm[3] |
|
|
Lattice volume (V) |
0.52996 nm3 | |
|
Formula units (Z) |
6 | |
|
Coordination geometry |
Octahedral (solid) Tetrahedral (liquid) |
|
|
Molecular shape |
Trigonal planar (monomeric vapour) |
|
| Thermochemistry | ||
|
Heat capacity (C) |
91.1 J/(mol·K)[4] | |
|
Std molar |
109.3 J/(mol·K)[4] | |
|
Std enthalpy of |
−704.2 kJ/mol[4] | |
|
Gibbs free energy (ΔfG⦵) |
−628.8 kJ/mol[4] | |
| Pharmacology | ||
|
ATC code |
D10AX01 (WHO) | |
| Hazards | ||
| GHS labelling:[6] | ||
|
Pictograms |

|
|
|
Signal word |
Danger | |
|
Hazard statements |
H314 | |
|
Precautionary statements |
P260, P280, P301+P330+P331, P303+P361+P353, P305+P351+P338+P310, P310 | |
| NFPA 704 (fire diamond) |
3 0 2 |
|
| Lethal dose or concentration (LD, LC): | ||
|
LD50 (median dose) |
380 mg/kg, rat (oral, anhydrous) 3311 mg/kg, rat (oral, hexahydrate) |
|
| NIOSH (US health exposure limits): | ||
|
PEL (Permissible) |
None[5] | |
|
REL (Recommended) |
2 mg/m3[5] | |
|
IDLH (Immediate danger) |
N.D.[5] | |
| Related compounds | ||
|
Other anions |
|
|
|
Other cations |
|
|
|
Related Lewis acids |
|
|
| Supplementary data page | ||
| Aluminium chloride (data page) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both are colourless crystals, but samples are often contaminated with iron(III) chloride, giving a yellow color.
The anhydrous material is important commercially. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry.[7] The compound is often cited as a Lewis acid. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature.
Structure[edit]
Anhydrous[edit]
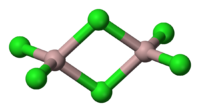
AlCl3 adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid AlCl3 has a sheet-like layered structure with cubic close-packed chloride ions. In this framework, the Al centres exhibit octahedral coordination geometry.[8] Yttrium(III) chloride adopts the same structure, as do a range of other compounds. When aluminium trichloride is in its melted state, it exists as the dimer Al2Cl6, with tetracoordinate aluminium. This change in structure is related to the lower density of the liquid phase (1.78 g/cm3) versus solid aluminium trichloride (2.48 g/cm3). Al2Cl6 dimers are also found in the vapour phase. At higher temperatures, the Al2Cl6 dimers dissociate into trigonal planar AlCl3 monomer, which is structurally analogous to BF3. The melt conducts electricity poorly,[9] unlike more ionic halides such as sodium chloride.
Aluminium chloride monomer belongs to the point group D3h in its monomeric form and D2h in its dimeric form.
Hexahydrate[edit]
The hexahydrate consists of octahedral [Al(H2O)6]3+ cation centers and chloride anions (Cl−) as counterions. Hydrogen bonds link the cation and anions.[10]
The hydrated form of aluminium chloride has an octahedral molecular geometry, with the central aluminium ion surrounded by six water ligand molecules. Being coordinatively saturated, the hydrate is of little value as a catalyst in Friedel-Crafts alkylation and related reactions.
Uses[edit]
Alkylation and acylation of arenes[edit]
AlCl3 is a common Lewis-acid catalyst for Friedel-Crafts reactions, both acylations and alkylations.[11] Important products are detergents and ethylbenzene. These types of reactions are the major use for aluminium chloride, for example, in the preparation of anthraquinone (used in the dyestuffs industry) from benzene and phosgene.[9] In the general Friedel-Crafts reaction, an acyl chloride or alkyl halide reacts with an aromatic system as shown:[11]
The alkylation reaction is more widely used than the acylation reaction, although its practice is more technically demanding. For both reactions, the aluminium chloride, as well as other materials and the equipment, should be dry, although a trace of moisture is necessary for the reaction to proceed.[12] Detailed procedures are available for alkylation[13] and acylation[14][15] of arenes.
A general problem with the Friedel-Crafts reaction is that the aluminium chloride catalyst sometimes is required in full stoichiometric quantities, because it complexes strongly with the products. This complication sometimes generates a large amount of corrosive waste. For these and similar reasons, the use of aluminium chloride has often been displaced by zeolites.[7]
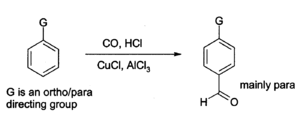
Aluminium chloride can also be used to introduce aldehyde groups onto aromatic rings, for example via the Gattermann-Koch reaction which uses carbon monoxide, hydrogen chloride and a copper(I) chloride co-catalyst.[16]
Other applications in organic and organometallic synthesis[edit]
Aluminium chloride finds a wide variety of other applications in organic chemistry.[17] For example, it can catalyse the «ene reaction», such as the addition of 3-buten-2-one (methyl vinyl ketone) to carvone:[18]
It is used to induce a variety of hydrocarbon couplings and rearrangements.[19][20]
Aluminium chloride combined with aluminium in the presence of an arene can be used to synthesize bis(arene) metal complexes, e.g. bis(benzene)chromium, from certain metal halides via the so-called Fischer-Hafner synthesis. Dichlorophenylphosphine is prepared by reaction of benzene and phosphorus trichloride catalyzed by aluminium chloride.[21]
Reactions[edit]
Anhydrous aluminium chloride is a powerful Lewis acid, capable of forming Lewis acid-base adducts with even weak Lewis bases such as benzophenone and mesitylene.[11] It forms tetrachloroaluminate ([AlCl4]−) in the presence of chloride ions.
Aluminium chloride reacts with calcium and magnesium hydrides in tetrahydrofuran forming tetrahydroaluminates.
Reactions with water[edit]
Anhydrous aluminium chloride is hygroscopic, having a very pronounced affinity for water. It fumes in moist air and hisses when mixed with liquid water as the Cl− ligands are displaced with H2O molecules to form the hexahydrate [Al(H2O)6]Cl3. The anhydrous phase cannot be regained on heating the hexahydrate. Instead HCl is lost leaving aluminium hydroxide or alumina (aluminium oxide):
- [Al(H2O)6]Cl3 → Al(OH)3 + 3 HCl + 3 H2O
Like metal aquo complexes, aqueous AlCl3 is acidic owing to the ionization of the aquo ligands:
- [Al(H2O)6]3+ ⇌ [Al(OH)(H2O)5]2+ + H+
Aqueous solutions behave similarly to other aluminium salts containing hydrated Al3+ ions, giving a gelatinous precipitate of aluminium hydroxide upon reaction with dilute sodium hydroxide:
- AlCl3 + 3 NaOH → Al(OH)3 + 3 NaCl
Synthesis[edit]
Aluminium chloride is manufactured on a large scale by the exothermic reaction of aluminium metal with chlorine or hydrogen chloride at temperatures between 650 to 750 °C (1,202 to 1,382 °F).[9]
- 2 Al + 3 Cl2 → 2 AlCl3
- 2 Al + 6 HCl → 2 AlCl3 + 3 H2
Aluminium chloride may be formed via a single displacement reaction between copper(II) chloride and aluminium metal.
- 2 Al + 3 CuCl2 → 2 AlCl3 + 3 Cu
In the US in 1993, approximately 21,000 tons were produced, not counting the amounts consumed in the production of aluminium.[7]
Hydrated aluminium trichloride is prepared by dissolving aluminium oxides in hydrochloric acid. Metallic aluminium also readily dissolves in hydrochloric acid ─ releasing hydrogen gas and generating considerable heat. Heating this solid does not produce anhydrous aluminium trichloride, the hexahydrate decomposes to aluminium hydroxide when heated:
- [Al(H2O)6]Cl3 → Al(OH)3 + 3 HCl + 3 H2O
Aluminium also forms a lower chloride, aluminium(I) chloride (AlCl), but this is very unstable and only known in the vapour phase.[9]
Natural occurrence[edit]
Anhydrous aluminium chloride is not found as a mineral. The hexahydrate, however, is known as the rare mineral chloraluminite.[22] A more complex, basic and hydrated aluminium chloride mineral is cadwaladerite.[23][22]
Safety[edit]
Anhydrous AlCl3 reacts vigorously with bases, so suitable precautions are required.
It can cause irritation to the eyes, skin, and the respiratory system if inhaled or on contact.[24]
See also[edit]
- Aluminium monochloride
References[edit]
- ^ a b c d Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.45. ISBN 1-4398-5511-0.
- ^ a b Aluminum chloride Archived 2014-05-05 at the Wayback Machine. Chemister.ru (2007-03-19). Retrieved on 2017-03-17.
- ^ a b Ketelaar, J. A. A. (1935). «Die Kristallstruktur der Aluminiumhalogenide II». Zeitschrift für Kristallographie – Crystalline Materials. 90 (1–6): 237–255. doi:10.1524/zkri.1935.90.1.237. S2CID 100796636.
- ^ a b c d Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 5.5. ISBN 1-4398-5511-0.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. «#0024». National Institute for Occupational Safety and Health (NIOSH).
- ^ Sigma-Aldrich Co., Aluminum chloride.
- ^ a b c Helmboldt, Otto; Keith Hudson, L.; Misra, Chanakya; Wefers, Karl; Heck, Wolfgang; Stark, Hans; Danner, Max; Rösch, Norbert (2007). «Aluminum Compounds, Inorganic». Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_527.pub2.
- ^ In contrast, AlBr3 has a more molecular structure, with the Al3+ centers occupying adjacent tetrahedral holes of the close-packed framework of Br− ions. Wells, A. F. (1984) Structural Inorganic Chemistry, Oxford Press, Oxford, United Kingdom. ISBN 0198553706.
- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 978-0-08-022057-4.
- ^ Andress, K.R.; Carpenter, C. (1934). «Kristallhydrate II. Die Struktur von Chromchlorid- und Aluminiumchloridhexahydrat». Zeitschrift für Kristallographie – Crystalline Materials. 87. doi:10.1524/zkri.1934.87.1.446.
- ^ a b c Olah, G. A., ed. (1963). Friedel-Crafts and Related Reactions. Vol. 1. New York City: Interscience.
- ^ Nenitzescu, Costin D.; Cantuniari, Ion P. (1933). «Durch Aluminiumchlorid Katalysierte Reaktion, VI. Mitteil.: Die Umlagerung des Cyclohexans in Metyl-cyclopentan». Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 66 (8): 1097–1100. doi:10.1002/cber.19330660817. ISSN 1099-0682.
- ^ Jonathan T. Reeves; Zhulin Tan; Daniel R. Fandrick; Jinhua J. Song; Nathan K. Yee; Chris H. Senanayake (2012). «Synthesis of Trifluoromethyl Ketones from Carboxylic Acids: 4-(3,4-Dibromophenyl)-1,1,1-trifluoro-4-methylpentan-2-one». Organic Syntheses. 89: 210. doi:10.15227/orgsyn.089.0210.
- ^ Kamil Paruch; Libor Vyklicky; Thomas J. Katz (2003). «Preparation of 9,10-Dimethoxyphenanthrene and 3,6-Diacetyl-9,10-Dimethoxyphenanthrene». Organic Syntheses. 80: 227. doi:10.15227/orgsyn.080.0227.
- ^ Alexander J. Seed; Vaishali Sonpatki; Mark R. Herbert (2002). «3-(4-Bromobenzoyl)propanoic Acid». Organic Syntheses. 79: 204. doi:10.15227/orgsyn.079.0204.
- ^ Wade, L. G. (2003) Organic Chemistry, 5th edition, Prentice Hall, Upper Saddle River, New Jersey, United States. ISBN 013033832X.
- ^ Galatsis, P. (1999) Handbook of Reagents for Organic Synthesis: Acidic and Basic Reagents, H. J. Reich, J. H. Rigby (eds.) Wiley, New York City. pp. 12–15. ISBN 978-0-471-97925-8.
- ^ Snider, B. B. (1980). «Lewis-acid catalyzed ene reactions». Acc. Chem. Res. 13 (11): 426. doi:10.1021/ar50155a007.
- ^ Reuben D. Rieke; Stephen E. Bales; Phillip M. Hudnall; Timothy P. Burns; Graham S. Poindexter (1979). «Highly Reactive Magnesium for the Preparation of Grignard Reagents: 1-Norbornanecarboxylic Acid». Organic Syntheses. 59: 85. doi:10.15227/orgsyn.059.0085.
- ^ Sami A. Shama; Carl C. Wamser (1983). «Hexamethyl Dewar Benzene». Organic Syntheses. 61: 62. doi:10.15227/orgsyn.061.0062.
- ^ B. Buchner; L. B. Lockhart Jr. (1951). «Phenyldichlorophosphine». Organic Syntheses. 31: 88. doi:10.15227/orgsyn.031.0088.
- ^ a b «List of Minerals». www.ima-mineralogy.org. International Mineralogical Association. March 21, 2011.
- ^ «Cadwaladerite». www.mindat.org.
- ^ Aluminum Chloride. solvaychemicals.us
External links[edit]
- International Chemical Safety Card 1125
- Index of Organic Synthesis procedures that utilize AlCl3
- The period 3 chlorides
- MSDS Archived 2011-07-22 at the Wayback Machine
- Government of Canada Fact Sheets and Frequently Asked Questions: Aluminum Salts
From Wikipedia, the free encyclopedia

Aluminium trichloride hexahydrate, pure (top), and contaminated with iron(III) chloride (bottom) |
||
|
||
| Names | ||
|---|---|---|
| IUPAC name
Aluminium chloride |
||
| Other names
Aluminium(III) chloride |
||
| Identifiers | ||
|
CAS Number |
|
|
|
3D model (JSmol) |
|
|
| ChEBI |
|
|
| ChemSpider |
|
|
| ECHA InfoCard | 100.028.371 |
|
| EC Number |
|
|
|
Gmelin Reference |
1876 | |
|
PubChem CID |
|
|
| RTECS number |
|
|
| UNII |
|
|
|
CompTox Dashboard (EPA) |
|
|
|
InChI
|
||
|
SMILES
|
||
| Properties | ||
|
Chemical formula |
AlCl3 | |
| Molar mass |
[1] |
|
| Appearance | Colourless crystals, hygroscopic | |
| Density |
[1] |
|
| Melting point |
|
|
|
Solubility in water |
|
|
| Solubility |
|
|
| Vapor pressure |
[2] |
|
| Viscosity |
[2] |
|
| Structure | ||
|
Crystal structure |
Monoclinic, mS16 | |
|
Space group |
C12/m1, No. 12[3] | |
|
Lattice constant |
a = 0.591 nm, b = 0.591 nm, c = 1.752 nm[3] |
|
|
Lattice volume (V) |
0.52996 nm3 | |
|
Formula units (Z) |
6 | |
|
Coordination geometry |
Octahedral (solid) Tetrahedral (liquid) |
|
|
Molecular shape |
Trigonal planar (monomeric vapour) |
|
| Thermochemistry | ||
|
Heat capacity (C) |
91.1 J/(mol·K)[4] | |
|
Std molar |
109.3 J/(mol·K)[4] | |
|
Std enthalpy of |
−704.2 kJ/mol[4] | |
|
Gibbs free energy (ΔfG⦵) |
−628.8 kJ/mol[4] | |
| Pharmacology | ||
|
ATC code |
D10AX01 (WHO) | |
| Hazards | ||
| GHS labelling:[6] | ||
|
Pictograms |

|
|
|
Signal word |
Danger | |
|
Hazard statements |
H314 | |
|
Precautionary statements |
P260, P280, P301+P330+P331, P303+P361+P353, P305+P351+P338+P310, P310 | |
| NFPA 704 (fire diamond) |
3 0 2 |
|
| Lethal dose or concentration (LD, LC): | ||
|
LD50 (median dose) |
380 mg/kg, rat (oral, anhydrous) 3311 mg/kg, rat (oral, hexahydrate) |
|
| NIOSH (US health exposure limits): | ||
|
PEL (Permissible) |
None[5] | |
|
REL (Recommended) |
2 mg/m3[5] | |
|
IDLH (Immediate danger) |
N.D.[5] | |
| Related compounds | ||
|
Other anions |
|
|
|
Other cations |
|
|
|
Related Lewis acids |
|
|
| Supplementary data page | ||
| Aluminium chloride (data page) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both are colourless crystals, but samples are often contaminated with iron(III) chloride, giving a yellow color.
The anhydrous material is important commercially. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry.[7] The compound is often cited as a Lewis acid. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature.
Structure[edit]
Anhydrous[edit]
AlCl3 adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid AlCl3 has a sheet-like layered structure with cubic close-packed chloride ions. In this framework, the Al centres exhibit octahedral coordination geometry.[8] Yttrium(III) chloride adopts the same structure, as do a range of other compounds. When aluminium trichloride is in its melted state, it exists as the dimer Al2Cl6, with tetracoordinate aluminium. This change in structure is related to the lower density of the liquid phase (1.78 g/cm3) versus solid aluminium trichloride (2.48 g/cm3). Al2Cl6 dimers are also found in the vapour phase. At higher temperatures, the Al2Cl6 dimers dissociate into trigonal planar AlCl3 monomer, which is structurally analogous to BF3. The melt conducts electricity poorly,[9] unlike more ionic halides such as sodium chloride.
Aluminium chloride monomer belongs to the point group D3h in its monomeric form and D2h in its dimeric form.
Hexahydrate[edit]
The hexahydrate consists of octahedral [Al(H2O)6]3+ cation centers and chloride anions (Cl−) as counterions. Hydrogen bonds link the cation and anions.[10]
The hydrated form of aluminium chloride has an octahedral molecular geometry, with the central aluminium ion surrounded by six water ligand molecules. Being coordinatively saturated, the hydrate is of little value as a catalyst in Friedel-Crafts alkylation and related reactions.
Uses[edit]
Alkylation and acylation of arenes[edit]
AlCl3 is a common Lewis-acid catalyst for Friedel-Crafts reactions, both acylations and alkylations.[11] Important products are detergents and ethylbenzene. These types of reactions are the major use for aluminium chloride, for example, in the preparation of anthraquinone (used in the dyestuffs industry) from benzene and phosgene.[9] In the general Friedel-Crafts reaction, an acyl chloride or alkyl halide reacts with an aromatic system as shown:[11]
The alkylation reaction is more widely used than the acylation reaction, although its practice is more technically demanding. For both reactions, the aluminium chloride, as well as other materials and the equipment, should be dry, although a trace of moisture is necessary for the reaction to proceed.[12] Detailed procedures are available for alkylation[13] and acylation[14][15] of arenes.
A general problem with the Friedel-Crafts reaction is that the aluminium chloride catalyst sometimes is required in full stoichiometric quantities, because it complexes strongly with the products. This complication sometimes generates a large amount of corrosive waste. For these and similar reasons, the use of aluminium chloride has often been displaced by zeolites.[7]
Aluminium chloride can also be used to introduce aldehyde groups onto aromatic rings, for example via the Gattermann-Koch reaction which uses carbon monoxide, hydrogen chloride and a copper(I) chloride co-catalyst.[16]
Other applications in organic and organometallic synthesis[edit]
Aluminium chloride finds a wide variety of other applications in organic chemistry.[17] For example, it can catalyse the «ene reaction», such as the addition of 3-buten-2-one (methyl vinyl ketone) to carvone:[18]
It is used to induce a variety of hydrocarbon couplings and rearrangements.[19][20]
Aluminium chloride combined with aluminium in the presence of an arene can be used to synthesize bis(arene) metal complexes, e.g. bis(benzene)chromium, from certain metal halides via the so-called Fischer-Hafner synthesis. Dichlorophenylphosphine is prepared by reaction of benzene and phosphorus trichloride catalyzed by aluminium chloride.[21]
Reactions[edit]
Anhydrous aluminium chloride is a powerful Lewis acid, capable of forming Lewis acid-base adducts with even weak Lewis bases such as benzophenone and mesitylene.[11] It forms tetrachloroaluminate ([AlCl4]−) in the presence of chloride ions.
Aluminium chloride reacts with calcium and magnesium hydrides in tetrahydrofuran forming tetrahydroaluminates.
Reactions with water[edit]
Anhydrous aluminium chloride is hygroscopic, having a very pronounced affinity for water. It fumes in moist air and hisses when mixed with liquid water as the Cl− ligands are displaced with H2O molecules to form the hexahydrate [Al(H2O)6]Cl3. The anhydrous phase cannot be regained on heating the hexahydrate. Instead HCl is lost leaving aluminium hydroxide or alumina (aluminium oxide):
- [Al(H2O)6]Cl3 → Al(OH)3 + 3 HCl + 3 H2O
Like metal aquo complexes, aqueous AlCl3 is acidic owing to the ionization of the aquo ligands:
- [Al(H2O)6]3+ ⇌ [Al(OH)(H2O)5]2+ + H+
Aqueous solutions behave similarly to other aluminium salts containing hydrated Al3+ ions, giving a gelatinous precipitate of aluminium hydroxide upon reaction with dilute sodium hydroxide:
- AlCl3 + 3 NaOH → Al(OH)3 + 3 NaCl
Synthesis[edit]
Aluminium chloride is manufactured on a large scale by the exothermic reaction of aluminium metal with chlorine or hydrogen chloride at temperatures between 650 to 750 °C (1,202 to 1,382 °F).[9]
- 2 Al + 3 Cl2 → 2 AlCl3
- 2 Al + 6 HCl → 2 AlCl3 + 3 H2
Aluminium chloride may be formed via a single displacement reaction between copper(II) chloride and aluminium metal.
- 2 Al + 3 CuCl2 → 2 AlCl3 + 3 Cu
In the US in 1993, approximately 21,000 tons were produced, not counting the amounts consumed in the production of aluminium.[7]
Hydrated aluminium trichloride is prepared by dissolving aluminium oxides in hydrochloric acid. Metallic aluminium also readily dissolves in hydrochloric acid ─ releasing hydrogen gas and generating considerable heat. Heating this solid does not produce anhydrous aluminium trichloride, the hexahydrate decomposes to aluminium hydroxide when heated:
- [Al(H2O)6]Cl3 → Al(OH)3 + 3 HCl + 3 H2O
Aluminium also forms a lower chloride, aluminium(I) chloride (AlCl), but this is very unstable and only known in the vapour phase.[9]
Natural occurrence[edit]
Anhydrous aluminium chloride is not found as a mineral. The hexahydrate, however, is known as the rare mineral chloraluminite.[22] A more complex, basic and hydrated aluminium chloride mineral is cadwaladerite.[23][22]
Safety[edit]
Anhydrous AlCl3 reacts vigorously with bases, so suitable precautions are required.
It can cause irritation to the eyes, skin, and the respiratory system if inhaled or on contact.[24]
See also[edit]
- Aluminium monochloride
References[edit]
- ^ a b c d Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.45. ISBN 1-4398-5511-0.
- ^ a b Aluminum chloride Archived 2014-05-05 at the Wayback Machine. Chemister.ru (2007-03-19). Retrieved on 2017-03-17.
- ^ a b Ketelaar, J. A. A. (1935). «Die Kristallstruktur der Aluminiumhalogenide II». Zeitschrift für Kristallographie – Crystalline Materials. 90 (1–6): 237–255. doi:10.1524/zkri.1935.90.1.237. S2CID 100796636.
- ^ a b c d Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 5.5. ISBN 1-4398-5511-0.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. «#0024». National Institute for Occupational Safety and Health (NIOSH).
- ^ Sigma-Aldrich Co., Aluminum chloride.
- ^ a b c Helmboldt, Otto; Keith Hudson, L.; Misra, Chanakya; Wefers, Karl; Heck, Wolfgang; Stark, Hans; Danner, Max; Rösch, Norbert (2007). «Aluminum Compounds, Inorganic». Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_527.pub2.
- ^ In contrast, AlBr3 has a more molecular structure, with the Al3+ centers occupying adjacent tetrahedral holes of the close-packed framework of Br− ions. Wells, A. F. (1984) Structural Inorganic Chemistry, Oxford Press, Oxford, United Kingdom. ISBN 0198553706.
- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 978-0-08-022057-4.
- ^ Andress, K.R.; Carpenter, C. (1934). «Kristallhydrate II. Die Struktur von Chromchlorid- und Aluminiumchloridhexahydrat». Zeitschrift für Kristallographie – Crystalline Materials. 87. doi:10.1524/zkri.1934.87.1.446.
- ^ a b c Olah, G. A., ed. (1963). Friedel-Crafts and Related Reactions. Vol. 1. New York City: Interscience.
- ^ Nenitzescu, Costin D.; Cantuniari, Ion P. (1933). «Durch Aluminiumchlorid Katalysierte Reaktion, VI. Mitteil.: Die Umlagerung des Cyclohexans in Metyl-cyclopentan». Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 66 (8): 1097–1100. doi:10.1002/cber.19330660817. ISSN 1099-0682.
- ^ Jonathan T. Reeves; Zhulin Tan; Daniel R. Fandrick; Jinhua J. Song; Nathan K. Yee; Chris H. Senanayake (2012). «Synthesis of Trifluoromethyl Ketones from Carboxylic Acids: 4-(3,4-Dibromophenyl)-1,1,1-trifluoro-4-methylpentan-2-one». Organic Syntheses. 89: 210. doi:10.15227/orgsyn.089.0210.
- ^ Kamil Paruch; Libor Vyklicky; Thomas J. Katz (2003). «Preparation of 9,10-Dimethoxyphenanthrene and 3,6-Diacetyl-9,10-Dimethoxyphenanthrene». Organic Syntheses. 80: 227. doi:10.15227/orgsyn.080.0227.
- ^ Alexander J. Seed; Vaishali Sonpatki; Mark R. Herbert (2002). «3-(4-Bromobenzoyl)propanoic Acid». Organic Syntheses. 79: 204. doi:10.15227/orgsyn.079.0204.
- ^ Wade, L. G. (2003) Organic Chemistry, 5th edition, Prentice Hall, Upper Saddle River, New Jersey, United States. ISBN 013033832X.
- ^ Galatsis, P. (1999) Handbook of Reagents for Organic Synthesis: Acidic and Basic Reagents, H. J. Reich, J. H. Rigby (eds.) Wiley, New York City. pp. 12–15. ISBN 978-0-471-97925-8.
- ^ Snider, B. B. (1980). «Lewis-acid catalyzed ene reactions». Acc. Chem. Res. 13 (11): 426. doi:10.1021/ar50155a007.
- ^ Reuben D. Rieke; Stephen E. Bales; Phillip M. Hudnall; Timothy P. Burns; Graham S. Poindexter (1979). «Highly Reactive Magnesium for the Preparation of Grignard Reagents: 1-Norbornanecarboxylic Acid». Organic Syntheses. 59: 85. doi:10.15227/orgsyn.059.0085.
- ^ Sami A. Shama; Carl C. Wamser (1983). «Hexamethyl Dewar Benzene». Organic Syntheses. 61: 62. doi:10.15227/orgsyn.061.0062.
- ^ B. Buchner; L. B. Lockhart Jr. (1951). «Phenyldichlorophosphine». Organic Syntheses. 31: 88. doi:10.15227/orgsyn.031.0088.
- ^ a b «List of Minerals». www.ima-mineralogy.org. International Mineralogical Association. March 21, 2011.
- ^ «Cadwaladerite». www.mindat.org.
- ^ Aluminum Chloride. solvaychemicals.us
External links[edit]
- International Chemical Safety Card 1125
- Index of Organic Synthesis procedures that utilize AlCl3
- The period 3 chlorides
- MSDS Archived 2011-07-22 at the Wayback Machine
- Government of Canada Fact Sheets and Frequently Asked Questions: Aluminum Salts
Aluminum Chloride is a combination of Aluminium and Chlorine elements. Aluminum is represented by Al. It is a lightweight silver metal that belongs to the boron family in the periodic table. Aluminum’s atomic number is 13. The density of it is lower than steel and had a greater affinity towards oxygen. So when aluminum is exposed to air, a thin protective layer of oxide is formed. On the other side Chlorine is a toxic, corrosive green-colored gas that causes irritation to the eyes and problems to the respiratory system. It is the second lightest member of the halogen family.
Aluminum Chloride is formed by an exothermic reaction between metallic aluminum and chlorine. Its chemical formula is AlCl3. Aluminum Chloride is also called Aluminium trichloride. The physical form of aluminum chloride is silvery-white colored powder but due to the presence of contaminants (ferric chloride), it acquires yellow color. It is soluble in various solvents such as water, chloroform, ethanol, CCl4, hydrogen chloride, and slightly soluble in benzene. It was corrosive and toxic.
Aluminum Chloride Structure
Aluminum Chloride Preparation
There are many ways of preparing AlCl3. All those processes are listed below along with equations.
- Aluminum Chloride is mainly produced by the exothermic reaction between aluminum and chlorine.
2Al + 3Cl2 —> 2AlCl3
- Aluminum Chloride can be formed by reacting the aluminum metal with hydrogen chloride.
2Al + 6HCl → 2AlCl3 + H2
- It was even prepared by conducting a single displacement reaction between copper chloride and aluminum metal.
2Al + 3CuCl2 → 2AlCl3 + 3Cu
Physical Properties of Aluminium Chloride
- Aluminum Chloride is a poor conductor of electricity in a molten state.
- The actual color of Aluminium Chloride is white in color but due to the presence of contaminants of Iron trichloride, it turns into yellowish color.
- It has a very low melting point and boiling point.
- Aluminum Chloride is in a liquid state when the temperature is above 190o C and pressure at above 2.5 atm.
Chemical Properties of Aluminium Chloride
- Aluminum Chloride is non-flammable, non-explosive.
- It is a corrosive solid.
- Aluminum chloride behaves violently when it reacts with water and bases.
- It is a lewis acid and an industrial catalyst.
Uses of Aluminium Chloride
- Used in pesticides & pharmaceuticals.
- Useful in manufacturing rubber, lubricants, paints, wood preservatives, and also petrochemicals like ethylbenzene.
- Aluminum Chloride is used in polymerization and isomerization reactions of light-weighted hydrocarbons like the production of ethylbenzene.
- Acts as a flux in melting aluminum.
- Aluminum Chloride is used as a catalyst for many chemical reactions. It is used in the preparation of anthraquinone from benzene and phosgene.
- It is used to attach aldehyde groups to aromatic series.
- Aluminum Chloride is mixed with aluminum, arene to synthesize arene metal complexes.
Sample Questions
Question 1: How does Aluminium Chloride react with water?
Answer:
Aluminium Chloride is hygroscopic which means it absorb moisture from air. Actually it fumes when in air containing moisture, creating a hissing sound when it comes contact with water. By this reaction Cl– ions are replaced with H2O and forms Hexahydrate i.e., Al(H2O)6Cl3. When heat is applied, ammonium hydroxide is obtained as final product by dissipating HCl.
Al(H2O)6Cl3 —> Al(OH)3 + 3HCl + 3H2O
Question 2: What are the melting and boiling points of Aluminium Chloride?
Answer:
The melting point of Aluminium Chloride is 192.6° C when it is anhydrous from. If it is a Hexahydrate then melting point is 100° C. The Boiling point of Aluminium Chloride is 180° C.
Question 3: Is Aluminium Chloride a Lewis Acid?
Answer:
Yes, Aluminium Chloride is a powerful Lewis acid. It forms Lewis Acid-base adducts even with bases that are weak in nature.
Example 1: Aluminium Chloride forms tetrachloroaluminate (AlCl4–) when Chlorine ions are present.
Example 2: AlCl3 react with magnesium and calcium hydride to form tetrahydridoaluminate.
Question 4: What is the molecular weight of Aluminium Chloride (AlCl3)?
Solution:
Molecular weight can be calculated by finding the weight of each element.
Weight of Aluminium = 26.982
Weight of chlorine = 35.453
Here there are three chlorine atoms so the above weight can be multiplied with 3 to give weight of 3 chlorine atoms.
Weight of 3 chlorine atoms = 3 × 35.453 = 106.359
So molecular weight of Aluminium chloride (AlCl3) = 26.982 + 106.359 = 133.341
Hence molecular weight of AlCl3 is 133.341 gram/molecule.
Question 5: Aluminium Chloride is hazardous?
Answer:
- Aluminium Chloride can explode when it comes contact to water. So precautions need to be taken like wearing gloves, face guards etc. It should be stored in tightly sealed container to protect it from moisture.
- It corrode metals like stainless steel.
- Exposure to AlCl3 for long period causes irritation to eyes and problem to respiratory organs.
- Aluminium Chloride is found to be neurotoxin and can destruct the nerve tissues. So from all these points, it can be concluded that AlCl3 is hazardous if proper precautions are not taken.
Question 6: What is the structure of Aluminium Chloride?
Answer:
The structure of Aluminium Chloride varies based on the factors such as state of compound i.e., solid, liquid or gaseous state and also the temperature it is exposed. In solid state, The structure of AlCl3 is Octahedral. In liquid/molten state, the structure is Tetrahedral. At higher temperature the structure is dissociates from tetrahedral to trigonal planar.
Aluminum Chloride is a combination of Aluminium and Chlorine elements. Aluminum is represented by Al. It is a lightweight silver metal that belongs to the boron family in the periodic table. Aluminum’s atomic number is 13. The density of it is lower than steel and had a greater affinity towards oxygen. So when aluminum is exposed to air, a thin protective layer of oxide is formed. On the other side Chlorine is a toxic, corrosive green-colored gas that causes irritation to the eyes and problems to the respiratory system. It is the second lightest member of the halogen family.
Aluminum Chloride is formed by an exothermic reaction between metallic aluminum and chlorine. Its chemical formula is AlCl3. Aluminum Chloride is also called Aluminium trichloride. The physical form of aluminum chloride is silvery-white colored powder but due to the presence of contaminants (ferric chloride), it acquires yellow color. It is soluble in various solvents such as water, chloroform, ethanol, CCl4, hydrogen chloride, and slightly soluble in benzene. It was corrosive and toxic.
Aluminum Chloride Structure
Aluminum Chloride Preparation
There are many ways of preparing AlCl3. All those processes are listed below along with equations.
- Aluminum Chloride is mainly produced by the exothermic reaction between aluminum and chlorine.
2Al + 3Cl2 —> 2AlCl3
- Aluminum Chloride can be formed by reacting the aluminum metal with hydrogen chloride.
2Al + 6HCl → 2AlCl3 + H2
- It was even prepared by conducting a single displacement reaction between copper chloride and aluminum metal.
2Al + 3CuCl2 → 2AlCl3 + 3Cu
Physical Properties of Aluminium Chloride
- Aluminum Chloride is a poor conductor of electricity in a molten state.
- The actual color of Aluminium Chloride is white in color but due to the presence of contaminants of Iron trichloride, it turns into yellowish color.
- It has a very low melting point and boiling point.
- Aluminum Chloride is in a liquid state when the temperature is above 190o C and pressure at above 2.5 atm.
Chemical Properties of Aluminium Chloride
- Aluminum Chloride is non-flammable, non-explosive.
- It is a corrosive solid.
- Aluminum chloride behaves violently when it reacts with water and bases.
- It is a lewis acid and an industrial catalyst.
Uses of Aluminium Chloride
- Used in pesticides & pharmaceuticals.
- Useful in manufacturing rubber, lubricants, paints, wood preservatives, and also petrochemicals like ethylbenzene.
- Aluminum Chloride is used in polymerization and isomerization reactions of light-weighted hydrocarbons like the production of ethylbenzene.
- Acts as a flux in melting aluminum.
- Aluminum Chloride is used as a catalyst for many chemical reactions. It is used in the preparation of anthraquinone from benzene and phosgene.
- It is used to attach aldehyde groups to aromatic series.
- Aluminum Chloride is mixed with aluminum, arene to synthesize arene metal complexes.
Sample Questions
Question 1: How does Aluminium Chloride react with water?
Answer:
Aluminium Chloride is hygroscopic which means it absorb moisture from air. Actually it fumes when in air containing moisture, creating a hissing sound when it comes contact with water. By this reaction Cl– ions are replaced with H2O and forms Hexahydrate i.e., Al(H2O)6Cl3. When heat is applied, ammonium hydroxide is obtained as final product by dissipating HCl.
Al(H2O)6Cl3 —> Al(OH)3 + 3HCl + 3H2O
Question 2: What are the melting and boiling points of Aluminium Chloride?
Answer:
The melting point of Aluminium Chloride is 192.6° C when it is anhydrous from. If it is a Hexahydrate then melting point is 100° C. The Boiling point of Aluminium Chloride is 180° C.
Question 3: Is Aluminium Chloride a Lewis Acid?
Answer:
Yes, Aluminium Chloride is a powerful Lewis acid. It forms Lewis Acid-base adducts even with bases that are weak in nature.
Example 1: Aluminium Chloride forms tetrachloroaluminate (AlCl4–) when Chlorine ions are present.
Example 2: AlCl3 react with magnesium and calcium hydride to form tetrahydridoaluminate.
Question 4: What is the molecular weight of Aluminium Chloride (AlCl3)?
Solution:
Molecular weight can be calculated by finding the weight of each element.
Weight of Aluminium = 26.982
Weight of chlorine = 35.453
Here there are three chlorine atoms so the above weight can be multiplied with 3 to give weight of 3 chlorine atoms.
Weight of 3 chlorine atoms = 3 × 35.453 = 106.359
So molecular weight of Aluminium chloride (AlCl3) = 26.982 + 106.359 = 133.341
Hence molecular weight of AlCl3 is 133.341 gram/molecule.
Question 5: Aluminium Chloride is hazardous?
Answer:
- Aluminium Chloride can explode when it comes contact to water. So precautions need to be taken like wearing gloves, face guards etc. It should be stored in tightly sealed container to protect it from moisture.
- It corrode metals like stainless steel.
- Exposure to AlCl3 for long period causes irritation to eyes and problem to respiratory organs.
- Aluminium Chloride is found to be neurotoxin and can destruct the nerve tissues. So from all these points, it can be concluded that AlCl3 is hazardous if proper precautions are not taken.
Question 6: What is the structure of Aluminium Chloride?
Answer:
The structure of Aluminium Chloride varies based on the factors such as state of compound i.e., solid, liquid or gaseous state and also the temperature it is exposed. In solid state, The structure of AlCl3 is Octahedral. In liquid/molten state, the structure is Tetrahedral. At higher temperature the structure is dissociates from tetrahedral to trigonal planar.
| Хлорид алюминия | |
 |
|
 |
|
| Общие | |
|---|---|
| Систематическое наименование | трихлоралюмин, трихлоралюминий, алюминия трихлорид |
| Традиционные названия | хлористый алюминий |
| Химическая формула | AlCl3 |
| Физические свойства | |
| Состояние (ст. усл.) | белые или бледно-желтые гигроскопичные твердые тела |
| Молярная масса | (ангидрид) 133.34 г/моль
(гексагидрат) 241.43 г/моль |
| Плотность | (ангидрид) 2.48 г/см³
(гексагидрат) 1.3 г/см³ |
| Термические свойства | |
| Температура плавления | (ангидрид) 192.4 °C |
| Температура кипения | (гексагидрат) 120 °C |
| Химические свойства | |
| Растворимость в воде | (0 °C) 43.9 г/100 мл
(10 °C) 44.9 г/100 мл (20 °C) 45.8 г/100 мл (30 °C) 46.6 г/100 мл (40 °C) 47.3 г/100 мл (60 °C) 48.1 г/100 мл (80 °C) 48.6 г/100 мл (100 °C) 49 г/100 мл |
| Растворимость в остальных веществах | растворим в хлороводороде, этиловом спирте, хлороформе; слабо растворим в бензоле г/100 мл |
| Структура | |
| Координационная геометрия | октаэдрическая (линейные)
четырехгранная (жидкость) |
| Кристаллическая структура | моноклинная |
| Классификация | |
| Рег. номер CAS | 7446-70-0,
10124-27-3 (гексагидрат) |
| SMILES | Cl[Al](Cl)Cl |
| RTECS | BD0530000 |
| Безопасность | |
| ЛД50 | ангидрид; крысы, орально: 380 мг/кг
гексагидрат; крысы, орально: 3311 мг/кг |
| Токсичность |  |
Хлорид алюминия (хлористый алюминий) — соль алюминия и соляной кислоты. Химическая формула — AlCl3.
Свойства
При обычном давлении возгоняется при 183 °C (под давлением плавится при 192,6 °C). В воде хорошо растворим (44,38 г в 100 г H2O при 25 °C); вследствие гидролиза дымит во влажном воздухе, выделяя HCl. Из водных растворов выпадает кристаллогидрат AlCl3· 6H2O — желтовато-белые расплывающиеся кристаллы. Хорошо растворим во многих органических соединениях (в этаноле — 100 г в 100 г спирта при 25 °C, в ацетоне, дихлорэтане, диэтиленгликоле, нитробензоле, тетрахлоруглероде и др.); однако практически не растворяется в бензоле и толуоле.
Получение
Важнейший способ получения хлорида алюминия в промышленности — действие смеси Cl2 и CO на обезвоженный каолин или боксит в шахтных печах:
- Al2O3 + ЗСО + ЗСl2 → 2AlCl3 + 3CO2
При температуре в 900 °C трихлорид бора и фосфид алюминия дают на выходе фосфид бора и хлорид алюминия:
Также есть и другие способы получения хлорида алюминия:
- Al + FeCl3 → AlCl3 + Fe
- Al(OH)3 + 3HCl → AlCl3 + 3H2O
- 3CuCl2 + 2Al → 2AlCl3 + 3Cu↓
- 2Al + 6HCl → 2AlCl3 + 3H2↑
Применение
Безводный хлорид алюминия образует продукты присоединения со многими неорганическими (например, NH3, H2S, SO2) и органическими (хлорангидриды кислот, эфиры и др.) веществами, с чем связано важнейшее техническое применение AlCl3 как катализатора при переработке нефти и при органических синтезах (например, реакция Фриделя — Крафтса). Гексагидрат и его растворы используются при очистке сточных вод, обработке древесины, производстве антиперспирантов и пр.
|
Хлорид алюминия |
|
|---|---|
| Систематическое наименование |
трихлоралюмин, трихлоралюминий, алюминия трихлорид |
| Традиционные названия | хлористый алюминий |
| Хим. формула | AlCl₃ |
| Состояние | белые или бледно-желтые гигроскопичные твердые кристаллы |
| Молярная масса | (ангидрид) 133.34 г/моль
(гексагидрат) 241,43 |
| Плотность | (ангидрид) 2,48 г/см³
(гексагидрат) 1,3 г/см³ |
| Т. плав. | (ангидрид) 192,4 ℃ |
| Т. кип. | (гексагидрат) 120 ℃ |
| Растворимость в воде | (0 °C) 43,9 г/100 мл
(10 °C) 44,9 г/100 мл (20 °C) 45,8 г/100 мл (30 °C) 46,6 г/100 мл (40 °C) 47,3 г/100 мл (60 °C) 48,1 г/100 мл (80 °C) 48,6 г/100 мл (100 °C) 49 г/100 мл |
| Растворимость в остальных веществах | растворим в хлороводороде, этиловом спирте, хлороформе слабо растворим в бензоле |
| Координационная геометрия | октаэдрическая (линейные)
четырехгранная (жидкость) |
| Кристаллическая структура | моноклинная сингония |
| Номер CAS | 7446-70-0 |
| PubChem | 24012 |
| ChemSpider | 22445 |
| Номер EINECS | 231-208-1 |
| RTECS | BD0530000 |
| ChEBI | 30114 |
| DrugBank | DB11081 |
| Номер ООН | 3264 |
| ЛД50 | ангидрид крысы, перорально: 380 мг/кг гексагидрат |
| Токсичность |  |
| Приводятся данные для стандартных условий (25 ℃, 100 кПа), если не указано иное. |
Хлорид алюминия (хлористый алюминий) — неорганическое соединение, соль алюминия и соляной кислоты с химической формулой AlCl3.
Свойства
В безводном виде бесцветные кристаллы, дымящие вследствие гидролиза во влажном воздухе, выделяя HCl[1]. При обычном давлении возгоняется при 183 °C (под давлением плавится при 192,6 °C).
В воде хорошо растворим (44,38 г в 100 г H2O при 25 °C). Из водных растворов выпадает в виде кристаллогидрата AlCl3·6H2O — желтовато-белые расплывающиеся на воздухе кристаллы. Хорошо растворим во многих органических соединениях (в этаноле — 100 г в 100 г спирта при 25 °C, в ацетоне, 1,2-дихлорэтане, этиленгликоле, нитробензоле, тетрахлоруглероде и др.); практически не растворяется в бензоле и толуоле.
Получение
Важнейший способ получения хлорида алюминия в промышленности — действие смеси Cl2 и CO на обезвоженный каолин или боксит в шахтных печах:
-
- Al2O3 + 3 CO + 3 Cl2 → 2 AlCl3 + 3 CO2
При температуре в 900 °C трихлорид бора и фосфид алюминия образуют фосфид бора и хлорид алюминия:
-
- BCl3 + AlP →900oC BP + AlCl3
Также есть и другие способы получения хлорида алюминия:
-
- Al + FeCl3 → AlCl3 + Fe
-
- Al(OH)3 + 3 HCl → AlCl3 + 3 H2O
-
- 3 CuCl2 + 2 Al → 2 AlCl3 + 3 C u
-
- 2 Al + 6 HCl → 2 AlCl3 + 3 H2
Применение
Безводный хлорид алюминия образует аддукты со многими неорганическими (например, NH3, H2S, SO2) и органическими (хлорангидриды кислот, эфиры и др.) веществами, с чем связано важнейшее техническое применение AlCl3 как катализатора при переработке нефти и при органических синтезах (например, реакция Фриделя — Крафтса).
Гексагидрат хлорида алюминия и его растворы используются при очистке сточных вод, обработке древесины, производстве антиперспирантов и пр.
Токсичность и безопасность
Хлорид алюминия токсичен при попадании в организм, а также обладает коррозионной активностью.
Поиск химических веществ по названиям или формулам.
Справочник содержит названия веществ и описания химических формул (в т.ч. структурные формулы и скелетные формулы).
Введите часть названия или формулу для поиска:
Языки:
По умолчанию |
Все возможные |
Из списка
|
Применить к найденному
Хлорид алюминия
Брутто-формула:
AlCl3
CAS# 7446-70-0
Категории:
Неорганические соли
PubChem CID: 24012
| ChemSpider ID: 22445
| CHEBI:30114
Названия
Русский:
- Хлорид алюминия [Wiki]
- алюминия трихлорид
- трихлоралюмин
- трихлоралюминий
- хлористый алюминий
English:
- ALUMINUM CHLORIDE
- Aluminium chloride [Wiki]
- Aluminium trichloride(IUPAC)
- Aluminum trichloride
- Aluminumchloride (8CI)
- EINECS:231-208-1
- Trichloroaluminum
- aluminium(III) chloride
- trichloroalumane(IUPAC)
Варианты формулы:
AlCl3
Al(+3)Cl(-1)3
Cl-Al<`|Cl>-Cl
ClAl<|Cl>/Cl
Вещества, имеющие отношение…
Анион:
Хлориды
Химический состав
Реакции, в которых участвует Хлорид алюминия
-
AlCl3 + 3NaOH -> Al(OH)3″|v» + 3NaCl
-
{M}{X}3 + 3{R}OH -> {M}(OH)3″|v» + 3{R}{X}
, где M =
Al Cr Fe Ti La; X =
Cl F Br I; R =
Li Na K Rb Cs NH4 -
Al(OH)3 + 3H{X} -> Al{X}3 + 3H2O
, где X =
F Cl Br I (NO3) -
{M}2O3 + 6H{X} = 2{M}{X}3 + 3H2O
, где M =
Fe Al Cr La Sc Y; X =
F Cl Br I (NO3) -
AlCl3 + 3K «T»-> 3KCl + Al
И ещё 22 реакции…