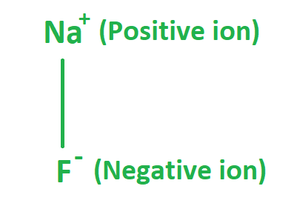From Wikipedia, the free encyclopedia

|
|

|
|
| Names | |
|---|---|
| Pronunciation | [1] |
| IUPAC name
Sodium fluoride |
|
| Other names
Florocid |
|
| Identifiers | |
|
CAS Number |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.028.789 |
| EC Number |
|
| KEGG |
|
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 1690 |
|
CompTox Dashboard (EPA) |
|
|
InChI
|
|
| Properties | |
|
Chemical formula |
NaF |
| Molar mass | 41.988173 g/mol |
| Appearance | White to greenish solid |
| Odor | odorless |
| Density | 2.558 g/cm3 |
| Melting point | 993 °C (1,819 °F; 1,266 K) |
| Boiling point | 1,704 °C (3,099 °F; 1,977 K) |
|
Solubility in water |
36.4 g/L (0 °C); 40.4 g/L (20 °C); 50.5 g/L (100 °C)[2] |
| Solubility | slightly soluble in HF, ammonia negligible in alcohol, acetone, SO2, dimethylformamide |
| Vapor pressure | 1 mmHg @ 1077 °C[3] |
|
Magnetic susceptibility (χ) |
−16.4·10−6 cm3/mol |
|
Refractive index (nD) |
1.3252 |
| Structure | |
|
Crystal structure |
Cubic |
|
Lattice constant |
a = 462 pm |
|
Molecular shape |
Octahedral |
| Thermochemistry | |
|
Heat capacity (C) |
46.82 J/(mol K) |
|
Std molar |
51.3 J/(mol K) |
|
Std enthalpy of |
-573.6 kJ/mol |
|
Gibbs free energy (ΔfG⦵) |
-543.3 kJ/mol |
| Pharmacology | |
|
ATC code |
A01AA01 (WHO) A12CD01 (WHO), V09IX06 (WHO) (18F) |
| Hazards | |
| GHS labelling: | |
|
Pictograms |
  
|
|
Signal word |
Danger |
|
Hazard statements |
H301, H315, H319, H335[4] |
| NFPA 704 (fire diamond) |
3 0 0 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose) |
52–130 mg/kg (oral in rats, mice, rabbits)[6] |
| NIOSH (US health exposure limits): | |
|
PEL (Permissible) |
TWA 2.5 mg/m3[5] |
|
REL (Recommended) |
TWA 2.5 mg/m3[5] |
|
IDLH (Immediate danger) |
250 mg/m3 (as F)[5] |
| Safety data sheet (SDS) | [4] |
| Related compounds | |
|
Other anions |
Sodium chloride Sodium bromide Sodium iodide Sodium astatide |
|
Other cations |
Lithium fluoride Potassium fluoride Rubidium fluoride Caesium fluoride Francium fluoride |
|
Related compounds |
TASF reagent |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Sodium fluoride (NaF) is an inorganic compound with the formula NaF. It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes and topical pharmaceuticals for the same purpose. In 2020, it was the 265th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[7][8] It is also used in metallurgy and in medical imaging.
Uses[edit]
Sodium fluoride is sold in tablets for cavity prevention
Dental caries[edit]
Fluoride salts are often added to municipal drinking water (as well as to certain food products in some countries) for the purpose of maintaining dental health. The fluoride enhances the strength of teeth by the formation of fluorapatite, a naturally occurring component of tooth enamel.[9][10][11] Although sodium fluoride is used to fluoridate water and is the standard by which other water-fluoridation compounds are gauged, hexafluorosilicic acid (H2SiF6) and its salt sodium hexafluorosilicate (Na2SiF6) are more commonly used additives in the United States.[12]
Osteoporosis[edit]
Fluoride supplementation has been extensively studied for the treatment of postmenopausal osteoporosis. This supplementation does not appear to be effective; even though sodium fluoride increases bone density, it does not decrease the risk of fractures.[13][14]
Medical imaging[edit]
In medical imaging, fluorine-18-labelled sodium fluoride (USP, sodium fluoride F18) is one of the oldest tracers used in positron emission tomography (PET), having been in use since the 1960s.[15] Relative to conventional bone scintigraphy carried out with gamma cameras or SPECT systems, PET offers more sensitivity and spatial resolution. Fluorine-18 has a half-life of 110 min, which requires it to be used promptly once produced; this logistical limitation hampered its adoption in the face of the more convenient technetium-99m-labelled radiopharmaceuticals. However fluorine-18 is generally considered to be a superior radiopharmaceutical for skeletal imaging. In particular it has a high and rapid bone uptake accompanied by very rapid blood clearance, which results in a high bone-to-background ratio in a short time.[16] Additionally the annihilation photons produced by decay of 18F have a high energy of 511 keV compared to the 140 keV photons of 99mTc.[17]
Chemistry[edit]
Sodium fluoride has a variety of specialty chemical applications in synthesis and extractive metallurgy. It reacts with electrophilic chlorides including acyl chlorides, sulfur chlorides, and phosphorus chloride.[18] Like other fluorides, sodium fluoride finds use in desilylation in organic synthesis. Sodium fluoride can be used to produce fluorocarbons via the Finkelstein reaction; this process has the advantage of being simple to perform on a small scale but is rarely used on an industrial scale due to the existence of more effective techniques (e.g. Electrofluorination, Fowler process).
Biology[edit]
Sodium fluoride is sometimes added at relatively high concentrations (~20 mM) to protein lysis buffers in order to inhibit endogenous phosphatases and thereby protect phosphorylated protein sites.[19] Sodium pyrophosphate and Sodium orthovanadate are also used for this purpose.[20]
Other uses[edit]
Sodium fluoride is used as a cleaning agent (e.g., as a «laundry sour»).[21]
Sodium fluoride can be used in a nuclear molten salt reactor.
Over a century ago,[when?] sodium fluoride was used as a stomach poison for plant-feeding insects.[22] Inorganic fluorides such as fluorosilicates and sodium fluoride complex magnesium ions as magnesium fluorophosphate. They inhibit enzymes such as enolase that require Mg2+ as a prosthetic group. Thus, fluoride poisoning prevents phosphate transfer in oxidative metabolism.[23]
Safety[edit]
The lethal dose for a 70 kg (154 lb) human is estimated at 5–10 g.[21]
Fluorides, particularly aqueous solutions of sodium fluoride, are rapidly and quite extensively absorbed by the human body.[24]
Fluorides interfere with electron transport and calcium metabolism. Calcium is essential for maintaining cardiac membrane potentials and in regulating coagulation. High ingestion of fluoride salts or hydrofluoric acid may result in fatal arrhythmias due to profound hypocalcemia. Chronic over-absorption can cause hardening of bones, calcification of ligaments, and buildup on teeth. Fluoride can cause irritation or corrosion to eyes, skin, and nasal membranes.[25]
Sodium fluoride is classed as toxic by both inhalation (of dusts or aerosols) and ingestion.[26] In high enough doses, it has been shown to affect the heart and circulatory system. For occupational exposures, the Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health have established occupational exposure limits at 2.5 mg/m3 over an eight-hour time-weighted average.[27]
In the higher doses used to treat osteoporosis, plain sodium fluoride can cause pain in the legs and incomplete stress fractures when the doses are too high; it also irritates the stomach, sometimes so severely as to cause peptic ulcer disease. Slow-release and enteric-coated versions of sodium fluoride do not have significant gastric side effects, and have milder and less frequent complications in the bones.[28] In the lower doses used for water fluoridation, the only clear adverse effect is dental fluorosis, which can alter the appearance of children’s teeth during tooth development; this is mostly mild and is unlikely to represent any real effect on aesthetic appearance or on public health.[29] A chronic fluoride ingestion of 1 ppm of fluoride in drinking water can cause mottling of the teeth (fluorosis) and an exposure of 1.7 ppm will produce mottling in 30%–50% of patients.[24]
Chemical structure[edit]
Sodium fluoride is an inorganic ionic compound, dissolving in water to give separated Na+ and F− ions. Like sodium chloride, it crystallizes in a cubic motif where both Na+ and F− occupy octahedral coordination sites;[30][31] its lattice spacing, approximately 462 pm, is smaller than that of sodium chloride (564 pm).
Occurrence[edit]
The mineral form of NaF, villiaumite, is moderately rare. It is known from plutonic nepheline syenite rocks.[32]
Production[edit]
NaF is prepared by neutralizing hydrofluoric acid or hexafluorosilicic acid (H2SiF6), both byproducts of the reaction of fluorapatite (Ca5(PO4)3F) from phosphate rock during the production of superphosphate fertilizer. Neutralizing agents include sodium hydroxide and sodium carbonate. Alcohols are sometimes used to precipitate the NaF:
- HF + NaOH → NaF + H2O
From solutions containing HF, sodium fluoride precipitates as the bifluoride salt sodium bifluoride (NaHF2). Heating the latter releases HF and gives NaF.
- HF + NaF ⇌ NaHF2
In a 1986 report, the annual worldwide consumption of NaF was estimated to be several million tonnes.[21]
See also[edit]
- Cryolite
- Fluoride therapy
References[edit]
- ^ Wells, John C. (2008), Longman Pronunciation Dictionary (3rd ed.), Longman, pp. 313 and 755, ISBN 978-1-4058-8118-0. According to this source, an alternative pronunciation of the second word is and, in the UK, also .
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. p. 5.194. ISBN 978-1-4398-5511-9.
- ^ Lewis, R.J. Sax’s Dangerous Properties of Industrial Materials. 10th ed. Volumes 1–3 New York, NY: John Wiley & Sons Inc., 1999., p. 3248
- ^ a b Sigma-Aldrich Co., Sodium Fluoride.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. «#0563». National Institute for Occupational Safety and Health (NIOSH).
- ^ Martel, B.; Cassidy, K. (2004), Chemical Risk Analysis: A Practical Handbook, Butterworth–Heinemann, p. 363, ISBN 978-1-903996-65-2
- ^ «The Top 300 of 2020». ClinCalc. Retrieved 7 October 2022.
- ^ «Sodium Fluoride – Drug Usage Statistics». ClinCalc. Retrieved 7 October 2022.
- ^ Bourne, volume editor, Geoffrey H. (1986). Dietary research and guidance in health and disease. Basel: Karger. p. 153. ISBN 978-3-8055-4341-5.
- ^ Klein, Cornelis (1999). Hurlbut, Cornelius S. (ed.). Manual of Mineralogy (after James D. Dana) (21st ed., rev. ed.). New York: J. Wiley. ISBN 978-0-471-31266-6.
- ^ Selwitz, Robert H; Ismail, Amid I; Pitts, Nigel B (January 2007). «Dental caries». The Lancet. 369 (9555): 51–59. doi:10.1016/S0140-6736(07)60031-2. PMID 17208642. S2CID 204616785.
- ^ Division of Oral Health, National Center for Prevention Services, CDC (1993), Fluoridation census 1992 (PDF), retrieved 2008-12-29.
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Haguenauer, D; Welch, V; Shea, B; Tugwell, P; Wells, G (2000). «Fluoride for treating postmenopausal osteoporosis». The Cochrane Database of Systematic Reviews. 2010 (4): CD002825. doi:10.1002/14651858.CD002825. PMC 8453489. PMID 11034769.
- ^ Vestergaard, P; Jorgensen, NR; Schwarz, P; Mosekilde, L (March 2008). «Effects of treatment with fluoride on bone mineral density and fracture risk—a meta-analysis». Osteoporosis International. 19 (3): 257–68. doi:10.1007/s00198-007-0437-6. PMID 17701094. S2CID 25890845.
- ^ Blau, Monte; Ganatra, Ramanik; Bender, Merrill A. (January 1972). «18F-fluoride for bone imaging». Seminars in Nuclear Medicine. 2 (1): 31–37. doi:10.1016/S0001-2998(72)80005-9. PMID 5059349.
- ^ Ordonez, A. A.; DeMarco, V. P.; Klunk, M. H.; Pokkali, S.; Jain, S.K. (October 2015). «Imaging Chronic Tuberculous Lesions Using Sodium [18F]Fluoride Positron Emission Tomography in Mice». Molecular Imaging and Biology. 17 (5): 609–614. doi:10.1007/s11307-015-0836-6. PMC 4561601. PMID 25750032.
- ^ Grant, F. D.; Fahey, F. H.; Packard, A. B.; Davis, R. T.; Alavi, A.; Treves, S. T. (12 December 2007). «Skeletal PET with 18F-Fluoride: Applying New Technology to an Old Tracer». Journal of Nuclear Medicine. 49 (1): 68–78. doi:10.2967/jnumed.106.037200. PMID 18077529.
- ^ Halpern, D.F. (2001), «Sodium Fluoride», Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, doi:10.1002/047084289X.rs071, ISBN 978-0-471-93623-7
- ^ Somerville, Laura L.; Wang, Kuan (1988). «Sarcomere matrix of striated muscle: In vivo phosphorylation of titin and nebulin in mouse diaphragm muscle». Archives of Biochemistry and Biophysics. Elsevier BV. 262 (1): 118–129. doi:10.1016/0003-9861(88)90174-9. ISSN 0003-9861. PMID 3355162.
- ^ «Overview of Protease and Phosphatase Inhibition for Protein Preparation — US». Thermo Fisher Scientific. 2017-05-10. Retrieved 2023-02-03.
- ^ a b c Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, René; Cuer, Jean Pierre (2000). «Fluorine Compounds, Inorganic». Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_307.
- ^ House, James E.; House, Kathleen A. (2015-09-10). Descriptive Inorganic Chemistry. Academic Press. p. 397. ISBN 978-0-12-802979-4.
- ^ Metcalf, Robert L. (2007), «Insect Control», Ullmann’s Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 9
- ^ a b Kapp, Robert (2005), «Fluorine», Encyclopedia of Toxicology, vol. 2 (2nd ed.), Elsevier, pp. 343–346
- ^ Greene Shepherd (2005), «Fluoride», Encyclopedia of Toxicology, vol. 2 (2nd ed.), Elsevier, pp. 342–343
- ^ NaF MSDS. hazard.com
- ^ CDC – NIOSH Pocket Guide to Chemical Hazards
- ^ Murray TM, Ste-Marie LG (1996). «Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada. 7. Fluoride therapy for osteoporosis». CMAJ. 155 (7): 949–54. PMC 1335460. PMID 8837545.
- ^ National Health and Medical Research Council (Australia) (2007). A systematic review of the efficacy and safety of fluoridation (PDF). ISBN 978-1-86496-415-8. Summary: Yeung CA (2008). «A systematic review of the efficacy and safety of fluoridation». Evid Based Dent. 9 (2): 39–43. doi:10.1038/sj.ebd.6400578. PMID 18584000.
- ^ Wells, A.F. (1984), Structural Inorganic Chemistry, Oxford: Clarendon Press, ISBN 978-0-19-855370-0
- ^ «Chemical and physical information», Toxicological profile for fluorides, hydrogen fluoride, and fluorine (PDF), Agency for Toxic Substances and Disease Registry (ATDSR), September 2003, p. 187, retrieved 2008-11-01
- ^ Mineral Handbook (PDF), Mineral Data Publishing, 2005.
External links[edit]
- «Sodium fluoride». Drug Information Portal. U.S. National Library of Medicine.
From Wikipedia, the free encyclopedia

|
|

|
|
| Names | |
|---|---|
| Pronunciation | [1] |
| IUPAC name
Sodium fluoride |
|
| Other names
Florocid |
|
| Identifiers | |
|
CAS Number |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.028.789 |
| EC Number |
|
| KEGG |
|
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 1690 |
|
CompTox Dashboard (EPA) |
|
|
InChI
|
|
| Properties | |
|
Chemical formula |
NaF |
| Molar mass | 41.988173 g/mol |
| Appearance | White to greenish solid |
| Odor | odorless |
| Density | 2.558 g/cm3 |
| Melting point | 993 °C (1,819 °F; 1,266 K) |
| Boiling point | 1,704 °C (3,099 °F; 1,977 K) |
|
Solubility in water |
36.4 g/L (0 °C); 40.4 g/L (20 °C); 50.5 g/L (100 °C)[2] |
| Solubility | slightly soluble in HF, ammonia negligible in alcohol, acetone, SO2, dimethylformamide |
| Vapor pressure | 1 mmHg @ 1077 °C[3] |
|
Magnetic susceptibility (χ) |
−16.4·10−6 cm3/mol |
|
Refractive index (nD) |
1.3252 |
| Structure | |
|
Crystal structure |
Cubic |
|
Lattice constant |
a = 462 pm |
|
Molecular shape |
Octahedral |
| Thermochemistry | |
|
Heat capacity (C) |
46.82 J/(mol K) |
|
Std molar |
51.3 J/(mol K) |
|
Std enthalpy of |
-573.6 kJ/mol |
|
Gibbs free energy (ΔfG⦵) |
-543.3 kJ/mol |
| Pharmacology | |
|
ATC code |
A01AA01 (WHO) A12CD01 (WHO), V09IX06 (WHO) (18F) |
| Hazards | |
| GHS labelling: | |
|
Pictograms |
  
|
|
Signal word |
Danger |
|
Hazard statements |
H301, H315, H319, H335[4] |
| NFPA 704 (fire diamond) |
3 0 0 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose) |
52–130 mg/kg (oral in rats, mice, rabbits)[6] |
| NIOSH (US health exposure limits): | |
|
PEL (Permissible) |
TWA 2.5 mg/m3[5] |
|
REL (Recommended) |
TWA 2.5 mg/m3[5] |
|
IDLH (Immediate danger) |
250 mg/m3 (as F)[5] |
| Safety data sheet (SDS) | [4] |
| Related compounds | |
|
Other anions |
Sodium chloride Sodium bromide Sodium iodide Sodium astatide |
|
Other cations |
Lithium fluoride Potassium fluoride Rubidium fluoride Caesium fluoride Francium fluoride |
|
Related compounds |
TASF reagent |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Sodium fluoride (NaF) is an inorganic compound with the formula NaF. It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes and topical pharmaceuticals for the same purpose. In 2020, it was the 265th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[7][8] It is also used in metallurgy and in medical imaging.
Uses[edit]
Sodium fluoride is sold in tablets for cavity prevention
Dental caries[edit]
Fluoride salts are often added to municipal drinking water (as well as to certain food products in some countries) for the purpose of maintaining dental health. The fluoride enhances the strength of teeth by the formation of fluorapatite, a naturally occurring component of tooth enamel.[9][10][11] Although sodium fluoride is used to fluoridate water and is the standard by which other water-fluoridation compounds are gauged, hexafluorosilicic acid (H2SiF6) and its salt sodium hexafluorosilicate (Na2SiF6) are more commonly used additives in the United States.[12]
Osteoporosis[edit]
Fluoride supplementation has been extensively studied for the treatment of postmenopausal osteoporosis. This supplementation does not appear to be effective; even though sodium fluoride increases bone density, it does not decrease the risk of fractures.[13][14]
Medical imaging[edit]
In medical imaging, fluorine-18-labelled sodium fluoride (USP, sodium fluoride F18) is one of the oldest tracers used in positron emission tomography (PET), having been in use since the 1960s.[15] Relative to conventional bone scintigraphy carried out with gamma cameras or SPECT systems, PET offers more sensitivity and spatial resolution. Fluorine-18 has a half-life of 110 min, which requires it to be used promptly once produced; this logistical limitation hampered its adoption in the face of the more convenient technetium-99m-labelled radiopharmaceuticals. However fluorine-18 is generally considered to be a superior radiopharmaceutical for skeletal imaging. In particular it has a high and rapid bone uptake accompanied by very rapid blood clearance, which results in a high bone-to-background ratio in a short time.[16] Additionally the annihilation photons produced by decay of 18F have a high energy of 511 keV compared to the 140 keV photons of 99mTc.[17]
Chemistry[edit]
Sodium fluoride has a variety of specialty chemical applications in synthesis and extractive metallurgy. It reacts with electrophilic chlorides including acyl chlorides, sulfur chlorides, and phosphorus chloride.[18] Like other fluorides, sodium fluoride finds use in desilylation in organic synthesis. Sodium fluoride can be used to produce fluorocarbons via the Finkelstein reaction; this process has the advantage of being simple to perform on a small scale but is rarely used on an industrial scale due to the existence of more effective techniques (e.g. Electrofluorination, Fowler process).
Biology[edit]
Sodium fluoride is sometimes added at relatively high concentrations (~20 mM) to protein lysis buffers in order to inhibit endogenous phosphatases and thereby protect phosphorylated protein sites.[19] Sodium pyrophosphate and Sodium orthovanadate are also used for this purpose.[20]
Other uses[edit]
Sodium fluoride is used as a cleaning agent (e.g., as a «laundry sour»).[21]
Sodium fluoride can be used in a nuclear molten salt reactor.
Over a century ago,[when?] sodium fluoride was used as a stomach poison for plant-feeding insects.[22] Inorganic fluorides such as fluorosilicates and sodium fluoride complex magnesium ions as magnesium fluorophosphate. They inhibit enzymes such as enolase that require Mg2+ as a prosthetic group. Thus, fluoride poisoning prevents phosphate transfer in oxidative metabolism.[23]
Safety[edit]
The lethal dose for a 70 kg (154 lb) human is estimated at 5–10 g.[21]
Fluorides, particularly aqueous solutions of sodium fluoride, are rapidly and quite extensively absorbed by the human body.[24]
Fluorides interfere with electron transport and calcium metabolism. Calcium is essential for maintaining cardiac membrane potentials and in regulating coagulation. High ingestion of fluoride salts or hydrofluoric acid may result in fatal arrhythmias due to profound hypocalcemia. Chronic over-absorption can cause hardening of bones, calcification of ligaments, and buildup on teeth. Fluoride can cause irritation or corrosion to eyes, skin, and nasal membranes.[25]
Sodium fluoride is classed as toxic by both inhalation (of dusts or aerosols) and ingestion.[26] In high enough doses, it has been shown to affect the heart and circulatory system. For occupational exposures, the Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health have established occupational exposure limits at 2.5 mg/m3 over an eight-hour time-weighted average.[27]
In the higher doses used to treat osteoporosis, plain sodium fluoride can cause pain in the legs and incomplete stress fractures when the doses are too high; it also irritates the stomach, sometimes so severely as to cause peptic ulcer disease. Slow-release and enteric-coated versions of sodium fluoride do not have significant gastric side effects, and have milder and less frequent complications in the bones.[28] In the lower doses used for water fluoridation, the only clear adverse effect is dental fluorosis, which can alter the appearance of children’s teeth during tooth development; this is mostly mild and is unlikely to represent any real effect on aesthetic appearance or on public health.[29] A chronic fluoride ingestion of 1 ppm of fluoride in drinking water can cause mottling of the teeth (fluorosis) and an exposure of 1.7 ppm will produce mottling in 30%–50% of patients.[24]
Chemical structure[edit]
Sodium fluoride is an inorganic ionic compound, dissolving in water to give separated Na+ and F− ions. Like sodium chloride, it crystallizes in a cubic motif where both Na+ and F− occupy octahedral coordination sites;[30][31] its lattice spacing, approximately 462 pm, is smaller than that of sodium chloride (564 pm).
Occurrence[edit]
The mineral form of NaF, villiaumite, is moderately rare. It is known from plutonic nepheline syenite rocks.[32]
Production[edit]
NaF is prepared by neutralizing hydrofluoric acid or hexafluorosilicic acid (H2SiF6), both byproducts of the reaction of fluorapatite (Ca5(PO4)3F) from phosphate rock during the production of superphosphate fertilizer. Neutralizing agents include sodium hydroxide and sodium carbonate. Alcohols are sometimes used to precipitate the NaF:
- HF + NaOH → NaF + H2O
From solutions containing HF, sodium fluoride precipitates as the bifluoride salt sodium bifluoride (NaHF2). Heating the latter releases HF and gives NaF.
- HF + NaF ⇌ NaHF2
In a 1986 report, the annual worldwide consumption of NaF was estimated to be several million tonnes.[21]
See also[edit]
- Cryolite
- Fluoride therapy
References[edit]
- ^ Wells, John C. (2008), Longman Pronunciation Dictionary (3rd ed.), Longman, pp. 313 and 755, ISBN 978-1-4058-8118-0. According to this source, an alternative pronunciation of the second word is and, in the UK, also .
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. p. 5.194. ISBN 978-1-4398-5511-9.
- ^ Lewis, R.J. Sax’s Dangerous Properties of Industrial Materials. 10th ed. Volumes 1–3 New York, NY: John Wiley & Sons Inc., 1999., p. 3248
- ^ a b Sigma-Aldrich Co., Sodium Fluoride.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. «#0563». National Institute for Occupational Safety and Health (NIOSH).
- ^ Martel, B.; Cassidy, K. (2004), Chemical Risk Analysis: A Practical Handbook, Butterworth–Heinemann, p. 363, ISBN 978-1-903996-65-2
- ^ «The Top 300 of 2020». ClinCalc. Retrieved 7 October 2022.
- ^ «Sodium Fluoride – Drug Usage Statistics». ClinCalc. Retrieved 7 October 2022.
- ^ Bourne, volume editor, Geoffrey H. (1986). Dietary research and guidance in health and disease. Basel: Karger. p. 153. ISBN 978-3-8055-4341-5.
- ^ Klein, Cornelis (1999). Hurlbut, Cornelius S. (ed.). Manual of Mineralogy (after James D. Dana) (21st ed., rev. ed.). New York: J. Wiley. ISBN 978-0-471-31266-6.
- ^ Selwitz, Robert H; Ismail, Amid I; Pitts, Nigel B (January 2007). «Dental caries». The Lancet. 369 (9555): 51–59. doi:10.1016/S0140-6736(07)60031-2. PMID 17208642. S2CID 204616785.
- ^ Division of Oral Health, National Center for Prevention Services, CDC (1993), Fluoridation census 1992 (PDF), retrieved 2008-12-29.
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Haguenauer, D; Welch, V; Shea, B; Tugwell, P; Wells, G (2000). «Fluoride for treating postmenopausal osteoporosis». The Cochrane Database of Systematic Reviews. 2010 (4): CD002825. doi:10.1002/14651858.CD002825. PMC 8453489. PMID 11034769.
- ^ Vestergaard, P; Jorgensen, NR; Schwarz, P; Mosekilde, L (March 2008). «Effects of treatment with fluoride on bone mineral density and fracture risk—a meta-analysis». Osteoporosis International. 19 (3): 257–68. doi:10.1007/s00198-007-0437-6. PMID 17701094. S2CID 25890845.
- ^ Blau, Monte; Ganatra, Ramanik; Bender, Merrill A. (January 1972). «18F-fluoride for bone imaging». Seminars in Nuclear Medicine. 2 (1): 31–37. doi:10.1016/S0001-2998(72)80005-9. PMID 5059349.
- ^ Ordonez, A. A.; DeMarco, V. P.; Klunk, M. H.; Pokkali, S.; Jain, S.K. (October 2015). «Imaging Chronic Tuberculous Lesions Using Sodium [18F]Fluoride Positron Emission Tomography in Mice». Molecular Imaging and Biology. 17 (5): 609–614. doi:10.1007/s11307-015-0836-6. PMC 4561601. PMID 25750032.
- ^ Grant, F. D.; Fahey, F. H.; Packard, A. B.; Davis, R. T.; Alavi, A.; Treves, S. T. (12 December 2007). «Skeletal PET with 18F-Fluoride: Applying New Technology to an Old Tracer». Journal of Nuclear Medicine. 49 (1): 68–78. doi:10.2967/jnumed.106.037200. PMID 18077529.
- ^ Halpern, D.F. (2001), «Sodium Fluoride», Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, doi:10.1002/047084289X.rs071, ISBN 978-0-471-93623-7
- ^ Somerville, Laura L.; Wang, Kuan (1988). «Sarcomere matrix of striated muscle: In vivo phosphorylation of titin and nebulin in mouse diaphragm muscle». Archives of Biochemistry and Biophysics. Elsevier BV. 262 (1): 118–129. doi:10.1016/0003-9861(88)90174-9. ISSN 0003-9861. PMID 3355162.
- ^ «Overview of Protease and Phosphatase Inhibition for Protein Preparation — US». Thermo Fisher Scientific. 2017-05-10. Retrieved 2023-02-03.
- ^ a b c Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, René; Cuer, Jean Pierre (2000). «Fluorine Compounds, Inorganic». Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_307.
- ^ House, James E.; House, Kathleen A. (2015-09-10). Descriptive Inorganic Chemistry. Academic Press. p. 397. ISBN 978-0-12-802979-4.
- ^ Metcalf, Robert L. (2007), «Insect Control», Ullmann’s Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 9
- ^ a b Kapp, Robert (2005), «Fluorine», Encyclopedia of Toxicology, vol. 2 (2nd ed.), Elsevier, pp. 343–346
- ^ Greene Shepherd (2005), «Fluoride», Encyclopedia of Toxicology, vol. 2 (2nd ed.), Elsevier, pp. 342–343
- ^ NaF MSDS. hazard.com
- ^ CDC – NIOSH Pocket Guide to Chemical Hazards
- ^ Murray TM, Ste-Marie LG (1996). «Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada. 7. Fluoride therapy for osteoporosis». CMAJ. 155 (7): 949–54. PMC 1335460. PMID 8837545.
- ^ National Health and Medical Research Council (Australia) (2007). A systematic review of the efficacy and safety of fluoridation (PDF). ISBN 978-1-86496-415-8. Summary: Yeung CA (2008). «A systematic review of the efficacy and safety of fluoridation». Evid Based Dent. 9 (2): 39–43. doi:10.1038/sj.ebd.6400578. PMID 18584000.
- ^ Wells, A.F. (1984), Structural Inorganic Chemistry, Oxford: Clarendon Press, ISBN 978-0-19-855370-0
- ^ «Chemical and physical information», Toxicological profile for fluorides, hydrogen fluoride, and fluorine (PDF), Agency for Toxic Substances and Disease Registry (ATDSR), September 2003, p. 187, retrieved 2008-11-01
- ^ Mineral Handbook (PDF), Mineral Data Publishing, 2005.
External links[edit]
- «Sodium fluoride». Drug Information Portal. U.S. National Library of Medicine.
NaF, бесцв. кристаллы с кубич. решеткой (а= 0,46344 нм, z= 4, пространств. группа Fm3m); т. пл. 996°С, т. кип. 1770°С; плотн. 2,766 г/см 3; C0p46,82 Дж/(моль . К); DH0 обр-576,6 кДж/моль, DG0 о6p— 542,6 кДж/моль, DH0 пл 34,25 кДж/моль, DH0 возг 280,7 кДж/моль (0 К), DH0 исп 176 кДж/моль; S029851,6 Дж/(моль . К). Раств. в воде (41,5 г/л при 20°С), безводном HF (30,1 г в 100 г при 11°С); DH0 растворения для бесконечно разб. водного р-ра 0,943 кДж/моль. Взаимод. с фторидами и оксифторидами металлов III-VIII гр., Be и Mg, образуя фторометаллаты; с HF и его водными р-рами дает NaHF2 (см. Гидрофториды металлов).
Встречается в природе в виде минерала виллиомита.
Н. ф. получают: гидролизом Na2SiF6 р-рами соды или щелочей с послед. отделением осадка от примеси кремне-геля; взаимод. Na2CO3, NaCl и др. солей Na с HF или NH4F в водном р-ре; термич. разложением NaHF2. Применяют Н. ф. в произ-ве Аl и HF (для получения Na3 [AlF6] и NaHF2), как компонент составов для очистки и алитирования металлов, флюсов для сварки, пайки и переплавки металлов, стекол, эмалей, керамики, огнеупоров, как компонент кислотоупорного цемента, термостойких смазок, составов для травления стекол, зубной пасты, твердых электролитов, как консервант древесины, инсектицид, реагент для фторирова-ния воды, сорбент для поглощения UF6 из газовых потоков и при очистке UF6 или WF5, реагент при получении фтор-углеводородов, как компонент спец. сортов бумаги, как ингибитор брожения, компонент огнезащитных составов и ср-в пожаротушения.
Мировое произ-во (без СССР) ок. 10 тыс. т/год. ПДК в воздухе рабочей зоны 0,2 мг/м 3.
Лит.: Антошкина И. Л., Меркулов В. А., в сб.: Химия и технология фтористых соединений. Труды УНИХИМ, в. 53, Свердловск, 1982, с. 9-23.
Э. Г. Раков.
Химическая энциклопедия. — М.: Советская энциклопедия.
.
1988.
Sodium is a chemical element with the atomic number 11 and the symbol Na (from Latin natrium). It’s a delicate, silvery-white metal with high reactivity. Sodium belongs to the periodic table’s group 1 as an alkali metal. It only has one stable isotope, 23Na. The free metal isn’t found in nature, thus it has to be made from compounds. Sodium is the sixth most prevalent element in the Earth’s crust, and it may be found in feldspars, sodalite, and rock salt, among other minerals (NaCl). Many sodium salts are very water-soluble: sodium ions have been leached from the Earth’s minerals by the action of water overages, and sodium and chlorine are the most prevalent dissolved elements in the seas by weight.
Sodium Fluoride
The inorganic compound sodium fluoride has the chemical formula of NaF. It’s utilised in trace quantities in drinking water fluoridation, toothpaste, and metallurgy. When sodium fluoride solutions are employed with hard water, calcium and magnesium fluoride insoluble compounds can develop. It’s a dry chemical that’s used to fluoridate drinking water, and it has to be manually weighed and added to the mixing tank. Villiaumite, a mineral form of NaF, is somewhat uncommon. It has been discovered in plutonic nepheline syenite rocks. It is slightly soluble in HF and ammonia but not in alcohol, acetone, SO2 and dimethylformamide.
Structure of Sodium Fluoride
The formula of Sodium Fluoride is made up of one fluoride anion (F–) and one sodium cation (Na+). Therefore, it has the molecular or chemical formula NaF. It has a molecular mass of 41.98 g/mol and crystallizes in a cubic motif, similar to sodium chloride, with both Na+ and F- occupying octahedral coordination sites; however, its lattice spacing, at 462 pm, is somewhat less than sodium chlorides.
Physical Properties of Sodium Fluoride
- Sodium Fluoride is a white to greenish solid and has no odour at all.
- The density of Sodium Fluoride is 2.558 g/cm3.
- The melting point of Sodium Fluoride is 993o C.
- The boiling point of Sodium Fluoride is 1704o C.
- The molecular structure of Sodium Fluoride is octahedral and its crystal structure is cubic.
- The standard enthalpy of formation of Sodium Fluoride is −573.6 kJ·mol−1.
- The standard molar entropy of Sodium Fluoride is 144 J·mol−1 K−1.
- The heat capacity of Sodium Fluoride is 46.82 J/(mol K).
- The solubility of Sodium Fluoride in water is 36.4 g/L at 0oC, 40.4 g/L at 20oC and 50.5 g/L at 100o C.
- It is basic in nature with a pH of 8.
- The refractive index of Sodium Fluoride is 1.3252.
- The magnetic susceptibility of Sodium Fluoride is −16.4 × 10−6 cm3/mol.
- The vapour pressure of Sodium Fluoride is 1 mmHg at 1077o C.
Chemical Properties of Sodium Fluoride
- Hydrogen fluoride and sodium hydroxide are formed when sodium fluoride combines with water. The chemical formula is as follows:
HF + NaOH → NaF + H2O
- Sodium fluoride performs a displacement reaction with chlorine, resulting in sodium chloride and fluorine. The chemical formula is as follows:
NaF + Cl2 → NaCl + F2
Uses of Sodium Fluoride
- Sodium fluoride is utilised as a pesticide and has not been demonstrated to prevent tooth decay in children when taken systemically during pregnancy.
- It is used in the fluoridation of public water which further purifies the water.
- Sodium fluoride L-glutamic acid monosodium salt, a compound synthesized using Sodium fluoride is used to treat encephalopathies linked with liver problems.
- It has a number of specific chemical uses in synthesis and extractive metallurgy.
- Electrophilic chlorides, such as acyl chlorides, sulphur chlorides, and phosphorus chlorides, react with it.
- Sodium fluoride, like other fluorides, is used in organic synthesis for desilylation.
- It is used in the Finkelstein reaction to create fluorocarbons from sodium fluoride.
- It is utilised as a cleaning agent in the laundry industry.
- It finds its massive usage in the nuclear-molten salt reactor industries.
Sample Questions
Question 1. What is the process for the preparation of Sodium Fluoride?
Solution:
By neutralizing Hydrofluoric acid with basic salt of sodium hydroxide, sodium fluoride is produced.
HF + NaOH → NaF + H2O
Question 2. Why was sodium fluoride used as a stomach poison for plant-feeding insects?
Solution:
Inorganic fluorides, such as fluorosilicates and sodium fluoride, form magnesium fluorophosphate when they combine with magnesium ions. They stop enzymes like enolase from using Mg2+ as a prosthetic group. As a result, fluoride poisoning hinders the transfer of phosphate in oxidative metabolism. This acts as a poison for insects as they are not able to get the desired stuff from plant and die in the process.
Question 3. What is the use of sodium fluoride in the field of dentistry?
Solution:
Cavity prevention is accomplished with sodium fluoride. This keeps the teeth healthier and more resistant to decay-causing acids and germs. It is also used for the treatment of osteoporosis and otospongiosis in adults but its use is controversial and further studies are expected. Fluoride salts are often added to municipal drinking water (and, in certain countries, to certain food products) in order to preserve tooth health. Fluoride strengthens teeth by causing fluorapatite, a naturally occurring component of tooth enamel, to develop.
Question 4. Briefly explain how sodium fluoride is toxic to the human body.
Solution:
The aqueous solutions of sodium fluoride are promptly and extensively absorbed by the human body. Electron transport and calcium metabolism are both hampered by these substances. Fluoride salts or hydrofluoric acid taken in large amounts might cause deadly arrhythmias owing to significant hypocalcemia. Chronic overabsorption can result in bone hardening, ligament calcification, and plaque deposition on teeth. Eyes, skin, and nasal membranes can be irritated or corroded by fluoride. Sodium fluoride is classed as toxic by both inhalation (of dusts or aerosols) and ingestion. It has been demonstrated to damage the heart and circulatory system in high enough concentrations.
Question 5. How fluoride is obtained and what is its function in the human body?
Solution:
Fluoride is a substance found in all natural water sources across the planet. This, like iron and calcium, dissolves into groundwater, on which we rely for drinking water.
It is required in the human body for the preservation and solidification of our bones, as well as the prevention of tooth disease. Most typically it is used to treat teeth plaque, a moderate type of gum disease (gingivitis), and weak and brittle bones (osteoporosis).
Sodium is a chemical element with the atomic number 11 and the symbol Na (from Latin natrium). It’s a delicate, silvery-white metal with high reactivity. Sodium belongs to the periodic table’s group 1 as an alkali metal. It only has one stable isotope, 23Na. The free metal isn’t found in nature, thus it has to be made from compounds. Sodium is the sixth most prevalent element in the Earth’s crust, and it may be found in feldspars, sodalite, and rock salt, among other minerals (NaCl). Many sodium salts are very water-soluble: sodium ions have been leached from the Earth’s minerals by the action of water overages, and sodium and chlorine are the most prevalent dissolved elements in the seas by weight.
Sodium Fluoride
The inorganic compound sodium fluoride has the chemical formula of NaF. It’s utilised in trace quantities in drinking water fluoridation, toothpaste, and metallurgy. When sodium fluoride solutions are employed with hard water, calcium and magnesium fluoride insoluble compounds can develop. It’s a dry chemical that’s used to fluoridate drinking water, and it has to be manually weighed and added to the mixing tank. Villiaumite, a mineral form of NaF, is somewhat uncommon. It has been discovered in plutonic nepheline syenite rocks. It is slightly soluble in HF and ammonia but not in alcohol, acetone, SO2 and dimethylformamide.
Structure of Sodium Fluoride
The formula of Sodium Fluoride is made up of one fluoride anion (F–) and one sodium cation (Na+). Therefore, it has the molecular or chemical formula NaF. It has a molecular mass of 41.98 g/mol and crystallizes in a cubic motif, similar to sodium chloride, with both Na+ and F- occupying octahedral coordination sites; however, its lattice spacing, at 462 pm, is somewhat less than sodium chlorides.
Physical Properties of Sodium Fluoride
- Sodium Fluoride is a white to greenish solid and has no odour at all.
- The density of Sodium Fluoride is 2.558 g/cm3.
- The melting point of Sodium Fluoride is 993o C.
- The boiling point of Sodium Fluoride is 1704o C.
- The molecular structure of Sodium Fluoride is octahedral and its crystal structure is cubic.
- The standard enthalpy of formation of Sodium Fluoride is −573.6 kJ·mol−1.
- The standard molar entropy of Sodium Fluoride is 144 J·mol−1 K−1.
- The heat capacity of Sodium Fluoride is 46.82 J/(mol K).
- The solubility of Sodium Fluoride in water is 36.4 g/L at 0oC, 40.4 g/L at 20oC and 50.5 g/L at 100o C.
- It is basic in nature with a pH of 8.
- The refractive index of Sodium Fluoride is 1.3252.
- The magnetic susceptibility of Sodium Fluoride is −16.4 × 10−6 cm3/mol.
- The vapour pressure of Sodium Fluoride is 1 mmHg at 1077o C.
Chemical Properties of Sodium Fluoride
- Hydrogen fluoride and sodium hydroxide are formed when sodium fluoride combines with water. The chemical formula is as follows:
HF + NaOH → NaF + H2O
- Sodium fluoride performs a displacement reaction with chlorine, resulting in sodium chloride and fluorine. The chemical formula is as follows:
NaF + Cl2 → NaCl + F2
Uses of Sodium Fluoride
- Sodium fluoride is utilised as a pesticide and has not been demonstrated to prevent tooth decay in children when taken systemically during pregnancy.
- It is used in the fluoridation of public water which further purifies the water.
- Sodium fluoride L-glutamic acid monosodium salt, a compound synthesized using Sodium fluoride is used to treat encephalopathies linked with liver problems.
- It has a number of specific chemical uses in synthesis and extractive metallurgy.
- Electrophilic chlorides, such as acyl chlorides, sulphur chlorides, and phosphorus chlorides, react with it.
- Sodium fluoride, like other fluorides, is used in organic synthesis for desilylation.
- It is used in the Finkelstein reaction to create fluorocarbons from sodium fluoride.
- It is utilised as a cleaning agent in the laundry industry.
- It finds its massive usage in the nuclear-molten salt reactor industries.
Sample Questions
Question 1. What is the process for the preparation of Sodium Fluoride?
Solution:
By neutralizing Hydrofluoric acid with basic salt of sodium hydroxide, sodium fluoride is produced.
HF + NaOH → NaF + H2O
Question 2. Why was sodium fluoride used as a stomach poison for plant-feeding insects?
Solution:
Inorganic fluorides, such as fluorosilicates and sodium fluoride, form magnesium fluorophosphate when they combine with magnesium ions. They stop enzymes like enolase from using Mg2+ as a prosthetic group. As a result, fluoride poisoning hinders the transfer of phosphate in oxidative metabolism. This acts as a poison for insects as they are not able to get the desired stuff from plant and die in the process.
Question 3. What is the use of sodium fluoride in the field of dentistry?
Solution:
Cavity prevention is accomplished with sodium fluoride. This keeps the teeth healthier and more resistant to decay-causing acids and germs. It is also used for the treatment of osteoporosis and otospongiosis in adults but its use is controversial and further studies are expected. Fluoride salts are often added to municipal drinking water (and, in certain countries, to certain food products) in order to preserve tooth health. Fluoride strengthens teeth by causing fluorapatite, a naturally occurring component of tooth enamel, to develop.
Question 4. Briefly explain how sodium fluoride is toxic to the human body.
Solution:
The aqueous solutions of sodium fluoride are promptly and extensively absorbed by the human body. Electron transport and calcium metabolism are both hampered by these substances. Fluoride salts or hydrofluoric acid taken in large amounts might cause deadly arrhythmias owing to significant hypocalcemia. Chronic overabsorption can result in bone hardening, ligament calcification, and plaque deposition on teeth. Eyes, skin, and nasal membranes can be irritated or corroded by fluoride. Sodium fluoride is classed as toxic by both inhalation (of dusts or aerosols) and ingestion. It has been demonstrated to damage the heart and circulatory system in high enough concentrations.
Question 5. How fluoride is obtained and what is its function in the human body?
Solution:
Fluoride is a substance found in all natural water sources across the planet. This, like iron and calcium, dissolves into groundwater, on which we rely for drinking water.
It is required in the human body for the preservation and solidification of our bones, as well as the prevention of tooth disease. Most typically it is used to treat teeth plaque, a moderate type of gum disease (gingivitis), and weak and brittle bones (osteoporosis).
Содержание
- Структурная формула
- Русское название
- Английское название
- Латинское название вещества Натрия фторид
- Химическое название
- Брутто формула
- Фармакологическая группа вещества Натрия фторид
- Нозологическая классификация
- Код CAS
- Фармакологическое действие
- Характеристика
- Фармакология
- Применение вещества Натрия фторид
- Противопоказания
- Побочные действия вещества Натрия фторид
- Взаимодействие
- Передозировка
- Способ применения и дозы
- Меры предосторожности
- Особые указания
- Торговые названия с действующим веществом Натрия фторид
Структурная формула
Русское название
Натрия фторид
Английское название
Sodium fluoride
Латинское название вещества Натрия фторид
Natrii fluoridum (род. Natrii fluoridi)
Химическое название
Фторид натрия
Фармакологическая группа вещества Натрия фторид
Фармакологическое действие
—
восполняющее дефицит фтора, ингибирующее костную резорбцию, противокариозное.
Характеристика
Бесцветные кристаллы или белый кристаллический порошок, растворим в 25 частях воды.
Фармакология
Ионы фтора стабилизируют кальций в процессе минерализации, замещая гидроксильную группу в кристаллах апатитов с образованием плохо растворимого фторапатита (обусловливает плотность твердых тканей). Индуцирует остеогенез путем стимуляции остеобластов. Уменьшает резорбцию кости, повышает ее устойчивость к действию остеокластов, увеличивает костную массу. Способствует пенетрации ионов фтора в эмаль зуба, стимулирует созревание и обеспечивает прочность эмали, предупреждая развитие кариеса. Оказывает бактерицидное действие в отношении кариесогенной микрофлоры, уменьшает продукцию ею кислот, в частности молочной.
При приеме внутрь хорошо абсорбируется (93–97% ионов фтора) независимо от приема пищи. Cmax в плазме достигается через 4 ч. При любой дозе 50% поступившего фтора накапливается в твердых тканях зуба и костной ткани. Не участвующий в процессе минерализации фторид выводится почками.
Применение вещества Натрия фторид
Остеопороз: первичный (постменопаузальный, пресенильный, сенильный, идиопатический), стероидный (профилактика и лечение); локальные остеопатии, профилактика кариеса у детей и взрослых, при содержании в питьевой воде фторидов ниже 0,6 мг/мл.
Противопоказания
Для системного применения: выраженные нарушения функции почек или печени, обострение язвенной болезни желудка или двенадцатиперстной кишки, беременность, кормление грудью, детский возраст до 6 мес, 3 лет, 6 или 14 лет (в зависимости от лекарственной формы и дозы).
Побочные действия вещества Натрия фторид
При системном применении: диспептические явления, боли в нижних конечностях и суставах, повышенная утомляемость, слабость, головная боль, остеосклероз, эктопическая кальцификация (особенно при сочетании с витамином D или А), гипотиреоз, идиосинкразия, аллергические реакции (кожная сыпь и др.).
Взаимодействие
Ионы кальция, магния и алюминия замедляют всасывание (образуя плохо растворимые соединения). Антациды снижают эффективность (рекомендуется применять их за 2 ч до приема натрия фторида). Витамины А и D способствуют эктопической кальцификации.
Передозировка
Симптомы: слезотечение, гиперсаливация, тошнота, анорексия, кровянистая диарея и рвота, боли в животе, нижних конечностях и суставах, сужение зрачков, нарушение зрения, слабость, миастения, тремор, судороги, гипертермия, учащение пульса, гипотензия; возможен летальный исход (дыхательная недостаточность и остановка дыхания). При длительном поступлении — гипотиреоз, а в период формирования зубов — флюороз (нарушение процесса формирования и обызвествления эмали, появление желтых, коричневых и матовых пятен, испещренности, повышение хрупкости и стираемости зубов).
Лечение: немедленное введение избыточных количеств жидкости и кальция (раствор кальция глюконата или лактата, молоко) для осаждения фторидов, индукция рвоты, установка желудочного лаважа закисленной водой или 1% раствором кальция хлорида, назначение солевых слабительных (30 г натрия сульфата), в/в введение электролитов (20 мл 10–20% раствора глюконата кальция), витаминных препаратов, симптоматическая терапия, мониторинг уровня кальция в крови; возможен гемодиализ.
Способ применения и дозы
При остеопорозе внутрь по 0,015–0,02 г 3–4 раза в сутки ежедневно, длительно (1–4 года) постоянно или курсами — 3 мес лечение, 2–3 мес перерыв. Для профилактики стероидного остеопороза по 1 табл. в сутки. Для профилактики кариеса в течение всего периода формирования зубов: детям от 2 до 6 лет внутрь длительными курсами таблетки по 0,0011 г, старше 6 лет — по 0,0022 г 1 раз в сутки; старше 16 лет — полоскание рта 0,05–0,2% раствором после еды и чистки зубов.
Меры предосторожности
Прерывистый курс лечения рекомендуется при появлении артралгий на фоне постоянной терапии препаратом. Применение у детей до 6 лет требует специального контроля. У пациентов с заболеваниями крови в период терапии рекомендуется регулярное проведение анализов крови. Во время системного применения необходимо ежегодно проводить рентгеновское обследование для оценки эффективности терапии и решения вопроса о продолжении лечения, а также для раннего выявления флюороза. При выборе дозы препарата следует учитывать содержание фторидов в питьевой воде. Для достижения адекватной минерализации костной ткани следует дополнительно обеспечить поступление в организм кальция (1–1,5 г в сутки) и магния.
Особые указания
Повышение уровня щелочной фосфатазы свидетельствует о развитии эффекта.
Торговые названия с действующим веществом Натрия фторид
Содержание
- Физические свойства
- Нахождение в природе
- Получение
- Использование
- Физиологическое значение
Фторид нáтрия — неорганическое бинарное соединение с химической формулой NaF.Белое кристаллическое вещество.
| Фторид натрия | |
|---|---|
| Общие | |
| Систематическое наименование |
Фторид натрия |
| Традиционные названия | фторид натрия; фтористый натрий, виллиомит |
| Хим. формула | NaF |
| Рац. формула | NaF |
| Физические свойства | |
| Состояние | бесцветный твердый порошок без запаха |
| Молярная масса | 41,988713 г/моль |
| Плотность | 2,558 г/см³ |
| Термические свойства | |
| Температура | |
| • плавления | 993 °C |
| • кипения | 1695 °C |
| • вспышки | негорюч °C |
| Мол. теплоёмк. | 46,9 Дж/(моль·К) |
| Энтальпия | |
| • образования | -576,6 кДж/моль |
| Давление пара | 0 ± 1 мм рт.ст. |
| Химические свойства | |
| Растворимость | |
| • в воде | 4,13 г/100 мл |
| • в остальных веществах | растворим в HF, нерастворим в этаноле |
| Классификация | |
| Рег. номер CAS | 7681-49-4 |
| PubChem | 5235 |
| Рег. номер EINECS | 231-667-8 |
| SMILES |
[F-].[Na+] |
| InChI |
1S/FH.Na/h1H;/q;+1/p-1 PUZPDOWCWNUUKD-UHFFFAOYSA-M |
| RTECS | WB0350000 |
| ChEBI | 28741 |
| Номер ООН | 1690 |
| ChemSpider | 5045 |
| Безопасность | |
| ЛД50 | (орально: крысы, мыши, кролики) 52-135 мг/кг |
| Токсичность | Класс опасности 2 |
| Пиктограммы ECB |  |
| NFPA 704 |
Физические свойства
Фторид натрия — бесцветные кристаллы с кубической решеткой (a = 0,46344 нм, пространственная группа Fm3m, Z=4). Трудно растворим в воде. Хорошо растворяется в безводной плавиковой кислоте. Кристаллогидратов не образует.
Нахождение в природе
В природе существует в виде относительно редкого минерала виллиомита: карминово-красные, темно-вишневые, изредка бесцветные кристаллы, содержит NaF с незначительными примесями, месторождения в Северной Америке, Африке, Кольский полуостров.
Так же NaF встречается в магматических породах, входит в состав в нефелинового сиенита.
Получение
В промышленности фторид натрия получают щелочным гидролизом гексафторсиликатов:
Степень гидролиза невелика, так как константа последней реакции pK = 10,8.
Присоединяет HF с образованием дифторгидрата натрия:
При избытке HF образуются высшие гидрофториды натрия:
известны соединения для n = 1÷4.
Сильные нелетучие кислоты разрушают фторид натрия:
Насыщенный гидроксид лития благодаря плохой растворимости фторида лития разрушает фторид натрия:
Образовывает гексафторсиликаты и гексафторалюминаты:
Расплав фторида натрия является электролитом, следовательно его можно разложить электролизом на элементы:
Использование
Таблетки, содержащие фторид натрия (натриум флуоратум)
Фторид натрия и образующийся из него фторапатит используются для укрепления зубной эмали, которая и сама содержит фторапатит. Кроме добавления фтора в зубные пасты, производится фторирование питьевой воды. Зубная паста часто содержит фторид натрия, который необходим для предотвращения кариеса. Кроме того, фторид натрия используется как моющее средство. Используется в различных отраслях химической промышленности — при синтезе и в металлургии. Фторид натрия является реагентом при синтезе фреонов.
Натрия фторид используется для сохранения образцов тканей в биохимии и лекарственных тестирований; ионы фтора останавливают гликолиз. Натрия фторид часто используется вместе с иодоуксусной кислотой, которая ингибирует создание фермента альдолазы.
Натрия фторид используют как компонент составов для очистки и алитирования металлов, флюсов для сварки, пайки и переплавки металлов, стекол, эмалей, керамики, огнеупоров, как компонент кислотоупорного цемента, термостойких смазок, составов для травления стекол, твердых электролитов, как консервант древесины, инсектицид, сорбент для поглощения UF6 из газовых потоков, реагент при получении фторуглеводородов, как компонент специальных сортов бумаги, как ингибитор брожения, компонент огнезащитных составов и средств пожаротушения.
Также имелись данные, что фторид натрия(+1) использовался в пищепроме, однако его применение в этой сфере ограничили из-за токсичности (токсичность обусловлена фторидом).
Физиологическое значение
Фторид натрия относится к потенциально-опасным веществам для человека и млекопитающих. Он классифицируется как токсичное вещество при ингаляции (например, через пыль) или при приеме пищи в высоких дозах. Как было показано, при достаточно высоких дозах влияет на сердечно-сосудистую систему; смертельная доза для человека при весе 70 кг оценивается в 5—10 г. В больших дозах, когда нужно использовать фторид натрия для лечения остеопороза, может вызвать боль в ногах и перепады в артериальном давлении, когда дозы слишком высоки, то происходит раздражение желудка, иногда такое сильное, что это может вызвать язву. В микроскопических количествах фтористый натрий NaF используется для фторирования воды. При большой концентрации фтора (или при частом употреблении продуктов, жидкостей и тому подобных продуктов, содержащих фтор) может вызвать флюороз зубов, который может привести к потере зубов.
ПДК в воздухе рабочей зоны: 1 мг/м³ (максимальная разовая), 0,2 мг/м³ (средняя сменная) в пересчёте на фторид-ионы.
Натрия фторид относится ко II классу токсичности согласно ГОСТ 12.1.007-76.