| Хлорид цинка | |
|---|---|
 |
|
| Хим. формула | ZnCl₂ |
| Рац. формула | ZnCl2 |
| Плотность | 2,91 ± 0,01 г/см³ |
| Т. плав. | 554 ± 1 °F |
| Т. кип. | 1350 ± 1 °F |
| Давление пара | 0 ± 1 мм рт.ст. |
| ГОСТ | ГОСТ 4529-78 ГОСТ 7345-78 |
| Рег. номер CAS | 7646-85-7 |
| PubChem | 5727 |
| Рег. номер EINECS | 231-592-0 |
| SMILES |
Cl[Zn]Cl |
| InChI |
1S/2ClH.Zn/h2*1H;/q;;+2/p-2 JIAARYAFYJHUJI-UHFFFAOYSA-L |
| RTECS | ZH1400000 |
| ChEBI | 49976 |
| Номер ООН | 2331 |
| ChemSpider | 5525 |
| ЛД50 | 200 мг/кг |
| Приводятся данные для стандартных условий (25 °C, 100 кПа), если не указано иного. |
Хлорид цинка (хлористый цинк, дихлорид цинка, цинк хлористый паяльная кислота) — химическое соединение цинка с хлором, имеющее формулу ZnCl2.
Белые гигроскопичные кристаллы.
Содержание
- 1 Свойства
- 1.1 Физические свойства
- 1.2 Химические свойства
- 2 Получение
- 3 Применение
- 4 Токсичность
Свойства
Физические свойства
- Молекулярная масса: 136,2954
- Температура плавления: 318 °C
- Температура кипения: 732 °C
- Растворимость в воде при 20 °C: 79,8 %.
Химические свойства
Концентрированные растворы имеют кислую среду, так как в результате гидролиза в воде присутствуют ионы H+.
Получение
- растворение цинка или его окиси в соляной кислоте с последующим выпариванием раствора
- нагревание жидкого цинка в потоке хлора
Применение
- ситцепечатание
- изготовление зубных цементов
- антисептическая пропитка дерева (например, шпал)
- очистка поверхности металлов от оксидов перед пайкой
- компонент при производстве фибры
- рафинирование расплавов цинковых сплавов
- фракционный анализ угольных проб
- в гальванических элементах
Токсичность
Хлорид цинка высокотоксичен, сильный ирритант. При контакте с кожей вызывает химические ожоги. Особенно опасно попадание в глаза. После контакта с кожей необходимо немедленно удалить вещество с использованием мыла и большого количества воды. После контакта с роговицей промыть глаза большим количеством воды, использовать глазные капли.
Минимальная смертельная доза (ЛД50) — 200 мг/кг. Смертельная доза для человека орально — 3-5 г.
From Wikipedia, the free encyclopedia

|
|

|
|
| Names | |
|---|---|
| IUPAC name
Zinc chloride |
|
| Other names
Zinc(II) chloride |
|
| Identifiers | |
|
CAS Number |
|
|
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.028.720 |
| EC Number |
|
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 2331 |
|
CompTox Dashboard (EPA) |
|
|
InChI
|
|
|
SMILES
|
|
| Properties | |
|
Chemical formula |
ZnCl2 |
| Molar mass | 136.315 g/mol |
| Appearance | white crystalline solid hygroscopic and very deliquescent |
| Odor | odorless |
| Density | 2.907 g/cm3 |
| Melting point | 290 °C (554 °F; 563 K)[1] |
| Boiling point | 732 °C (1,350 °F; 1,005 K)[1] |
|
Solubility in water |
432.0 g/ 100 g (25 °C) |
| Solubility | soluble in ethanol, glycerol and acetone |
| Solubility in ethanol | 430.0 g/100ml |
|
Magnetic susceptibility (χ) |
−65.0·10−6 cm3/mol |
| Structure | |
|
Coordination geometry |
Tetrahedral, linear in the gas phase |
| Pharmacology | |
|
ATC code |
B05XA12 (WHO) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
|
Main hazards |
Moderately toxic, irritant[2] |
| GHS labelling: | |
|
Pictograms |
  
|
|
Signal word |
Danger |
|
Hazard statements |
H302, H314, H410 |
|
Precautionary statements |
P273, P280, P301+P330+P331, P305+P351+P338, P308+P310 |
| NFPA 704 (fire diamond) |
3 0 0 |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose) |
350 mg/kg (rat, oral) 350 mg/kg (mouse, oral) 200 mg/kg (guinea pig, oral) 1100 mg/kg (rat, oral) 1250 mg/kg (mouse, oral)[4] |
|
LC50 (median concentration) |
1260 mg/m3 (rat, 30 min) 1180 mg-min/m3[4] |
| NIOSH (US health exposure limits): | |
|
PEL (Permissible) |
TWA 1 mg/m3 (fume)[3] |
|
REL (Recommended) |
TWA 1 mg/m3 ST 2 mg/m3 (fume)[3] |
|
IDLH (Immediate danger) |
50 mg/m3 (fume)[3] |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
|
Other anions |
Zinc fluoride Zinc bromide Zinc iodide |
|
Other cations |
Cadmium chloride Mercury(II) chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water.[5] This salt is hygroscopic and even deliquescent. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.
Structure and properties[edit]
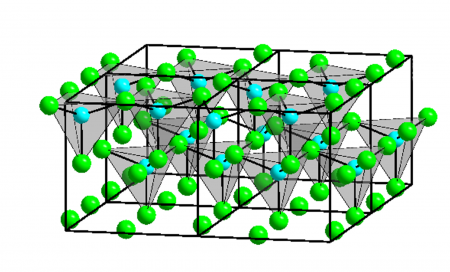
Four crystalline forms (polymorphs) of ZnCl2 are known: α, β, γ, and δ. Each case features tetrahedral Zn2+ centers.[6]
| Form | Symmetry | Pearson symbol | Group | No | a (nm) | b (nm) | c (nm) | Z | ρ (g/cm3) |
|---|---|---|---|---|---|---|---|---|---|
| α | tetragonal | tI12 | I42d | 122 | 0.5398 | 0.5398 | 0.64223 | 4 | 3.00 |
| β | tetragonal | tP6 | P42/nmc | 137 | 0.3696 | 0.3696 | 1.071 | 2 | 3.09 |
| γ | monoclinic | mP36 | P21/c | 14 | 0.654 | 1.131 | 1.23328 | 12 | 2.98 |
| δ | orthorhombic | oP12 | Pna21 | 33 | 0.6125 | 0.6443 | 0.7693 | 4 | 2.98 |
Here a, b, and c are lattice constants, Z is the number of structure units per unit cell, and ρ is the density calculated from the structure parameters.[7][8][9]
The orthorhombic form (δ) rapidly changes to one of the other forms on exposure to the atmosphere. A possible explanation is that the OH− ions originating from the absorbed water facilitate the rearrangement.[6] Rapid cooling of molten ZnCl2 gives a glass.[10]
Molten ZnCl2 has a high viscosity at its melting point and a comparatively low electrical conductivity, which increases markedly with temperature.[11][12] As indicated by a Raman scattering study, the viscosity is explained by the presence of polymers,[13]. Neutron scattering study indicated the presence of tetrahedral {ZnCl4} centers, which requires aggregation of ZnCl2 monomers as well..[14]
In the gas phase, ZnCl2 molecules are linear with a bond length of 205 pm.
Hydrates[edit]
Five hydrates of zinc chloride are known: ZnCl2(H2O)n with n = 1, 1.5, 2.5, 3 and 4.[15] The tetrahydrate ZnCl2(H2O)4 crystallizes from aqueous solutions of zinc chloride.[15]
Preparation and purification[edit]
Anhydrous ZnCl2 can be prepared from zinc and hydrogen chloride:
- Zn + 2 HCl → ZnCl2 + H2
Hydrated forms and aqueous solutions may be readily prepared similarly by treating Zn metal, zinc carbonate, zinc oxide, and zinc sulfide with hydrochloric acid:
- ZnS + 2 HCl + 4 H2O → ZnCl2(H2O)4 + H2S
Unlike many other elements, zinc essentially exists in only one oxidation state, 2+, which simplifies the purification of the chloride.
Commercial samples of zinc chloride typically contain water and products from hydrolysis as impurities. Such samples may be purified by recrystallization from hot dioxane. Anhydrous samples can be purified by sublimation in a stream of hydrogen chloride gas, followed by heating the sublimate to 400 °C in a stream of dry nitrogen gas.[16] Finally, the simplest method relies on treating the zinc chloride with thionyl chloride.[17]
Reactions[edit]
Molten anhydrous ZnCl2 at 500–700 °C dissolves zinc metal, and, on rapid cooling of the melt, a yellow diamagnetic glass is formed, which Raman studies indicate contains the Zn2+
2 ion.[15]
A number of salts containing the tetrachlorozincate anion, ZnCl2−
4, are known.[11] «Caulton’s reagent», V2Cl3(thf)6Zn2Cl6 is an example of a salt containing Zn2Cl2−
6.[18][19]
The compound Cs3ZnCl5 contains tetrahedral ZnCl2−
4 and Cl− anions.[6] No compounds containing the ZnCl4−
6 ion have been characterized.[6]
Zinc chloride dissolves readily in water to give ZnClxH2O(4−x) species and some free chloride.[20][21][22] Aqueous solutions of ZnCl2 are acidic: a 6 M aqueous solution has a pH of 1.[15] The acidity of aqueous ZnCl2 solutions relative to solutions of other Zn2+ salts (say the sulfate) is due to the formation of the tetrahedral chloro aqua complexes where the reduction in coordination number from 6 to 4 further reduces the strength of the O–H bonds in the solvated water molecules.[23]
In alkali solution, zinc chloride converts to various zinc hydroxychlorides. These include Zn(OH)3Cl2−, Zn(OH)2Cl2−
2, ZnOHCl2−
3, and the insoluble Zn5(OH)8Cl2·H2O. The latter is the mineral simonkolleite.[24] When zinc chloride hydrates are heated, HCl gas evolves and hydroxychlorides result.[25]
When solutions of zinc chloride are treated with ammonia, various complexes of «ammines» are produced. These include Zn(NH3)4Cl2·H2O and on concentration ZnCl2(NH3)2.[26] The former contains the Zn(NH3)62+ ion,[6] and the latter is molecular with a distorted tetrahedral geometry.[27] The species in aqueous solution have been investigated and show that Zn(NH3)42+ is the main species present with Zn(NH3)3Cl+ also present at lower NH3:Zn ratio.[28]
Aqueous zinc chloride reacts with zinc oxide to form an amorphous cement that was first investigated in 1855 by Stanislas Sorel. Sorel later went on to investigate the related magnesium oxychloride cement, which bears his name.[29]
When hydrated zinc chloride is heated, one obtains a residue of Zn(OH)Cl e.g.[30]
- ZnCl2·2H2O → ZnCl(OH) + HCl + H2O
The compound ZnCl2·1⁄2HCl·H2O may be prepared by careful precipitation from a solution of ZnCl2 acidified with HCl. It contains a polymeric anion (Zn2Cl5−)n with balancing monohydrated hydronium ions, H5O2+ ions.[6][31]
Cellulose dissolves in aqueous solutions of ZnCl2, and zinc-cellulose complexes have been detected.[32] Cellulose also dissolves in molten ZnCl2 hydrate and carboxylation and acetylation performed on the cellulose polymer.[33]
Thus, although many zinc salts have different formulas and different crystal structures, these salts behave very similarly in aqueous solution. For example, solutions prepared from any of the polymorphs of ZnCl2, as well as other halides (bromide, iodide), and the sulfate can often be used interchangeably for the preparation of other zinc compounds. Illustrative is the preparation of zinc carbonate:
- ZnCl2(aq) + Na2CO3(aq) → ZnCO3(s) + 2 NaCl(aq)
Role in organic chemistry[edit]
Zinc chloride is used as a catalyst or reagent in diverse reactions conducted on an industrial scale. The partial hydrolysis of benzal chloride in the presence of zinc chloride is the main route to benzoyl chloride. It serves as a catalyst for the production of methylene-bis(dithiocarbamate).[5]
The combination of hydrochloric acid and ZnCl2, known as the «Lucas reagent», is effective for the preparation of alkyl chlorides from alcohols. Similar reactions are the basis of industrial routes from methanol and ethanol respectively to methyl chloride and ethyl chloride.
Laboratory syntheses[edit]
Zinc chloride is a common reagent in the laboratory useful Lewis acid in organic chemistry.[34]
Molten zinc chloride catalyses the conversion of methanol to hexamethylbenzene:[35]
- 15 CH
3OH → C
6(CH
3)
6 + 3 CH
4 + 15 H
2O
Other examples include catalyzing (A) the Fischer indole synthesis,[36] and also (B) Friedel-Crafts acylation reactions involving activated aromatic rings[37][38]
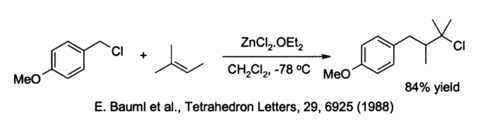
Related to the latter is the classical preparation of the dye fluorescein from phthalic anhydride and resorcinol, which involves a Friedel-Crafts acylation.[39] This transformation has in fact been accomplished using even the hydrated ZnCl2 sample shown in the picture above.
Zinc chloride also activates benzylic and allylic halides towards substitution by weak nucleophiles such as alkenes:[40]
In similar fashion, ZnCl2 promotes selective NaBH3CN reduction of tertiary, allylic or benzylic halides to the corresponding hydrocarbons.
Zinc chloride is also a useful starting reagent for the synthesis of many organozinc reagents, such as those used in the palladium catalyzed Negishi coupling with aryl halides or vinyl halides.[41] In such cases the organozinc compound is usually prepared by transmetallation from an organolithium or a Grignard reagent, for example:
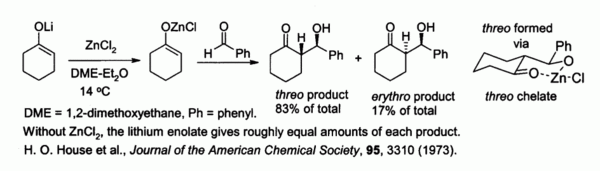
Zinc enolates, prepared from alkali metal enolates and ZnCl2, provide control of stereochemistry in aldol condensation reactions due to chelation on to the zinc. In the example shown below, the threo product was favored over the erythro by a factor of 5:1 when ZnCl2 in DME/ether was used.[42] The chelate is more stable when the bulky phenyl group is pseudo-equatorial rather than pseudo-axial, i.e., threo rather than erythro.
Other uses[edit]
As a metallurgical flux[edit]
The use of zinc chloride as a flux, sometimes in a mixture with ammonium chloride (see also Zinc ammonium chloride), involves the production of HCl and its subsequent reaction with surface oxides.
Zinc chloride reacts with metal oxides (MO) to give derivatives of the idealized formula MZnOCl2.[43][additional citation(s) needed] This reaction is relevant to the utility of ZnCl2 solution as a flux for soldering — it dissolves passivating oxides, exposing the clean metal surface.[43] Fluxes with ZnCl2 as an active ingredient are sometimes called «tinner’s fluid».
Zinc chloride forms two salts with ammonium chloride: (NH4)2ZnCl4 and (NH4)3ClZnCl4, which decompose on heating liberating HCl, just as zinc chloride hydrate does. The action of zinc chloride/ammonium chloride fluxes, for example, in the hot-dip galvanizing process produces H2 gas and ammonia fumes.[44]
In textile and paper processing[edit]
Concentrated aqueous solutions of zinc chloride (more than 64% weight/weight zinc chloride in water) have dissolving starch, silk, and cellulose.
Relevant to its affinity for these materials, ZnCl2 is used as a fireproofing agent and in fabric «refresheners» such as Febreze. Vulcanized fibre is made by soaking paper in concentrated zinc chloride.
Smoke grenades[edit]
The zinc chloride smoke mixture («HC») used in smoke grenades contains zinc oxide, hexachloroethane and granular aluminium powder, which, when ignited, react to form zinc chloride, carbon and aluminium oxide smoke, an effective smoke screen.[45]
Fingerprint detection[edit]
Ninhydrin reacts with amino acids and amines to form a colored compound «Ruhemann’s purple» (RP). Spraying with a zinc chloride solution forms a 1:1 complex RP:ZnCl(H2O)2, which is more readily detected as it fluoresces more intensely than RP.[46]
Disinfectant and wood preservative[edit]
Dilute aqueous zinc chloride was used as a disinfectant under the name «Burnett’s Disinfecting Fluid».
[47] From 1839 Sir William Burnett promoted its use as a disinfectant as well as a wood preservative.[48] The Royal Navy conducted trials into its use as a disinfectant in the late 1840s, including during the cholera epidemic of 1849; and at the same time experiments were conducted into its preservative properties as applicable to the shipbuilding and railway industries. Burnett had some commercial success with his eponymous fluid. Following his death however, its use was largely superseded by that of carbolic acid and other proprietary products.
Safety[edit]
Zinc chloride is a chemical irritant of the eyes, skin, and respiratory system.[5][49]
Additional reading[edit]
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- The Merck Index, 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960.
- D. Nicholls, Complexes and First-Row Transition Elements, Macmillan Press, London, 1973.
- J. March, Advanced Organic Chemistry, 4th ed., p. 723, Wiley, New York, 1992.
- G. J. McGarvey, in Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation, (R. M. Coates, S. E. Denmark, eds.), pp. 220–3, Wiley, New York, 1999.
References[edit]
- ^ a b O’Neil, M. J.; et al. (2001). The Merck index : an encyclopedia of chemicals, drugs, and biologicals. N. J.: Whitehouse Station. ISBN 978-0911910131.
- ^ Zinc chloride toxicity
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. «#0674». National Institute for Occupational Safety and Health (NIOSH).
- ^ a b «Zinc chloride fume». Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c Dieter M. M. Rohe; Hans Uwe Wolf (2007). «Zinc Compounds». Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–6. doi:10.1002/14356007.a28_537.
- ^ a b c d e f Wells, A. F. (1984). Structural Inorganic Chemistry. Oxford: Clarendon Press. ISBN 978-0-19-855370-0.
- ^ Oswald, H. R.; Jaggi, H. (1960). «Zur Struktur der wasserfreien Zinkhalogenide I. Die wasserfreien Zinkchloride». Helvetica Chimica Acta. 43 (1): 72–77. doi:10.1002/hlca.19600430109.
- ^ Brynestad, J.; Yakel, H. L. (1978). «Preparation and Structure of Anhydrous Zinc Chloride». Inorganic Chemistry. 17 (5): 1376–1377. doi:10.1021/ic50183a059.
- ^ Brehler, B. (1961). «Kristallstrukturuntersuchungen an ZnCl2«. Zeitschrift für Kristallographie. 115 (5–6): 373–402. Bibcode:1961ZK….115..373B. doi:10.1524/zkri.1961.115.5-6.373.
- ^ Mackenzie, J. D.; Murphy, W. K. (1960). «Structure of Glass-Forming Halides. II. Liquid Zinc Chloride». The Journal of Chemical Physics. 33 (2): 366–369. Bibcode:1960JChPh..33..366M. doi:10.1063/1.1731151.
- ^ a b Prince, R. H. (1994). King, R. B. (ed.). Encyclopedia of Inorganic Chemistry. John Wiley & Sons. ISBN 978-0-471-93620-6.
- ^ Ray, H. S. (2006). Introduction to Melts: Molten Salts, Slags and Glasses. Allied Publishers. ISBN 978-81-7764-875-1.
- ^ Danek, V. (2006). Physico-Chemical Analysis of Molten Electrolytes. Elsevier. ISBN 978-0-444-52116-3.
- ^ Price, D. L.; Saboungi, M.-L.; Susman, S.; Volin, K. J.; Wright, A. C. (1991). «Neutron Scattering Function of Vitreous and Molten Zinc Chloride». Journal of Physics: Condensed Matter. 3 (49): 9835–9842. Bibcode:1991JPCM….3.9835P. doi:10.1088/0953-8984/3/49/001. S2CID 250902741.
- ^ a b c d Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- ^ Glenn J. McGarvey Jean-François Poisson Sylvain Taillemaud (2016). «Zinc chloride». Encyclopedia of Reagents for Organic Synthesis: 1–20. doi:10.1002/047084289X.rz007.pub3. ISBN 9780470842898.
- ^ Pray, A. P. (1990). Anhydrous Metal Chlorides. Inorganic Syntheses. Vol. 28. pp. 321–322.
- ^ Mulzer, J.; Waldmann, H., eds. (1998). Organic Synthesis Highlights. Vol. 3. Wiley-VCH. ISBN 978-3-527-29500-5.
- ^ Bouma, R. J.; Teuben, J. H.; Beukema, W. R.; Bansemer, R. L.; Huffman, J. C.; Caulton, K. G. (1984). «Identification of the Zinc Reduction Product of VCl3 · 3THF as [V2Cl3(THF)6]2[Zn2Cl6]». Inorganic Chemistry. 23 (17): 2715–2718. doi:10.1021/ic00185a033.
- ^ Irish, D. E.; McCarroll, B.; Young, T. F. (1963). «Raman Study of Zinc Chloride Solutions». The Journal of Chemical Physics. 39 (12): 3436–3444. Bibcode:1963JChPh..39.3436I. doi:10.1063/1.1734212.
- ^ Yamaguchi, T.; Hayashi, S.; Ohtaki, H. (1989). «X-Ray Diffraction and Raman Studies of Zinc(II) Chloride Hydrate Melts, ZnCl2 · R H2O (R = 1.8, 2.5, 3.0, 4.0, and 6.2)». The Journal of Physical Chemistry. 93 (6): 2620–2625. doi:10.1021/j100343a074.
- ^ Pye, C. C.; Corbeil, C. R.; Rudolph, W. W. (2006). «An ab initio Investigation of Zinc Chloro Complexes». Physical Chemistry Chemical Physics. 8 (46): 5428–5436. Bibcode:2006PCCP….8.5428P. doi:10.1039/b610084h. ISSN 1463-9076. PMID 17119651. S2CID 37521287.
- ^ Brown, I. D. (2006). The Chemical Bond in Inorganic Chemistry: The Bond Valence Model. Oxford University Press. ISBN 978-0-19-929881-5.
- ^ Zhang, X. G. (1996). Corrosion and Electrochemistry of Zinc. Springer. ISBN 978-0-306-45334-2. Staff writer(s). «Simonkolleite Mineral Data». webmineral.com. Retrieved October 16, 2014.
- ^ Feigl, F.; Caldas, A. (1956). «Some Applications of Fusion Reactions with Zinc Chloride in Inorganic Spot Test Analysis». Microchimica Acta. 44 (7–8): 1310–1316. doi:10.1007/BF01257465. S2CID 96823985.
- ^ Vulte, H. T. (2007). Laboratory Manual of Inorganic Preparations. Read Books. ISBN 978-1-4086-0840-1.
- ^ Yamaguchi, T.; Lindqvist, O. (1981). «The Crystal Structure of Diamminedichlorozinc(II), ZnCl2(NH3)2. A New Refinement» (PDF). Acta Chemica Scandinavica A. 35 (9): 727–728. doi:10.3891/acta.chem.scand.35a-0727.
- ^ Yamaguchi, T.; Ohtaki, H. (1978). «X-Ray Diffraction Studies on the Structures of Tetraammine- and Triamminemonochlorozinc(II) Ions in Aqueous Solution». Bulletin of the Chemical Society of Japan. 51 (11): 3227–3231. doi:10.1246/bcsj.51.3227.
- ^ Wilson, A. D.; Nicholson, J. W. (1993). Acid-Base Cements: Their Biomedical and Industrial Applications. Cambridge University Press. ISBN 978-0-521-37222-0.
- ^ House, J. E. (2008). Inorganic Chemistry. Academic Press. ISBN 978-0-12-356786-4.
- ^ Mellow, J. W. (1946). A Comprehensive Treatise on Inorganic and Theoretical Chemistry. Longmans, Green.
- ^ Xu, Q.; Chen, L.-F. (1999). «Ultraviolet Spectra and Structure of Zinc-Cellulose Complexes in Zinc Chloride Solution». Journal of Applied Polymer Science. 71 (9): 1441–1446. doi:10.1002/(SICI)1097-4628(19990228)71:9<1441::AID-APP8>3.0.CO;2-G.
- ^ Fischer, S.; Leipner, H.; Thümmler, K.; Brendler, E.; Peters, J. (2003). «Inorganic Molten Salts as Solvents for Cellulose». Cellulose. 10 (3): 227–236. doi:10.1023/A:1025128028462. S2CID 92194004.
- ^ Olah, George A.; Doggweiler, Hans; Felberg, Jeff D.; Frohlich, Stephan; Grdina, Mary Jo; Karpeles, Richard; Keumi, Takashi; Inaba, Shin-ichi; Ip, Wai M.; Lammertsma, Koop; Salem, George; Tabor, Derrick (1984). «Onium Ylide chemistry. 1. Bifunctional acid-base-catalyzed conversion of heterosubstituted methanes into ethylene and derived hydrocarbons. The onium ylide mechanism of the C1 → C2 conversion». J. Am. Chem. Soc. 106 (7): 2143–2149. doi:10.1021/ja00319a039.
- ^ Chang, Clarence D. (1983). «Hydrocarbons from Methanol». Catal. Rev. — Sci. Eng. 25 (1): 1–118. doi:10.1080/01614948308078874.
- ^ Shriner, R. L.; Ashley, W. C.; Welch, E. (1942). «2-Phenylindole». Organic Syntheses. 22: 98. doi:10.15227/orgsyn.022.00981955.; Collective Volume, vol. 3, p. 725
- ^ Cooper, S. R. (1941). «Resacetophenone». Organic Syntheses. 21: 103. doi:10.15227/orgsyn.021.0103.; Collective Volume, vol. 3, p. 761
- ^ Dike, S. Y.; Merchant, J. R.; Sapre, N. Y. (1991). «A New and Efficient General Method for the Synthesis of 2-Spirobenzopyrans: First Synthesis of Cyclic Analogues of Precocene I and Related Compounds». Tetrahedron. 47 (26): 4775–4786. doi:10.1016/S0040-4020(01)86481-4.
- ^ Furnell, B. S. (1989). Vogel’s Textbook of Practical Organic Chemistry (5th ed.). New York: Longman/Wiley.
- ^ Bauml, E.; Tschemschlok, K.; Pock, R.; Mayr, H. (1988). «Synthesis of γ-Lactones from Alkenes Employing p-Methoxybenzyl Chloride as +CH2-CO2− Equivalent» (PDF). Tetrahedron Letters. 29 (52): 6925–6926. doi:10.1016/S0040-4039(00)88476-2.
- ^ Kim, S.; Kim, Y. J.; Ahn, K. H. (1983). «Selective Reduction of Tertiary, Allyl, and Benzyl Halides by Zinc-Modified Cyanoborohydride in Diethyl Ether». Tetrahedron Letters. 24 (32): 3369–3372. doi:10.1016/S0040-4039(00)86272-3.
- ^ House, H. O.; Crumrine, D. S.; Teranishi, A. Y.; Olmstead, H. D. (1973). «Chemistry of Carbanions. XXIII. Use of Metal Complexes to Control the Aldol Condensation». Journal of the American Chemical Society. 95 (10): 3310–3324. doi:10.1021/ja00791a039.
- ^ a b Wiberg, Nils (2007). Lehrbuch der Anorganischen Chemie [Holleman & Wiberg, Textbook of Inorganic chemistry] (in German). de Gruyter, Berlin. p. 1491. ISBN 978-3-11-017770-1.
- ^ American Society for Metals (1990). ASM handbook. ASM International. ISBN 978-0-87170-021-6.
- ^ Sample, B. E. (1997). Methods for Field Studies of Effects of Military Smokes, Obscurants, and Riot-control Agents on Threatened and Endangered Species. DIANE Publishing. ISBN 978-1-4289-1233-5.
- ^ Menzel, E. R. (1999). Fingerprint Detection with Lasers. CRC Press. ISBN 978-0-8247-1974-6.
- ^ Watts, H. (1869). A Dictionary of Chemistry and the Allied Branches of Other Sciences. Longmans, Green.
- ^ McLean, David (April 2010). «Protecting wood and killing germs: ‘Burnett’s Liquid’ and the origins of the preservative and disinfectant industries in early Victorian Britain». Business History. 52 (2): 285–305. doi:10.1080/00076791003610691. S2CID 154790730.
- ^ «NIOSH Pocket Guide to Chemical Hazards». CDC.gov. Retrieved 30 October 2020.
External links[edit]
- Grades and Applications of Zinc Chloride
- PubChem ZnCl2 summary.
From Wikipedia, the free encyclopedia

|
|

|
|
| Names | |
|---|---|
| IUPAC name
Zinc chloride |
|
| Other names
Zinc(II) chloride |
|
| Identifiers | |
|
CAS Number |
|
|
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.028.720 |
| EC Number |
|
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 2331 |
|
CompTox Dashboard (EPA) |
|
|
InChI
|
|
|
SMILES
|
|
| Properties | |
|
Chemical formula |
ZnCl2 |
| Molar mass | 136.315 g/mol |
| Appearance | white crystalline solid hygroscopic and very deliquescent |
| Odor | odorless |
| Density | 2.907 g/cm3 |
| Melting point | 290 °C (554 °F; 563 K)[1] |
| Boiling point | 732 °C (1,350 °F; 1,005 K)[1] |
|
Solubility in water |
432.0 g/ 100 g (25 °C) |
| Solubility | soluble in ethanol, glycerol and acetone |
| Solubility in ethanol | 430.0 g/100ml |
|
Magnetic susceptibility (χ) |
−65.0·10−6 cm3/mol |
| Structure | |
|
Coordination geometry |
Tetrahedral, linear in the gas phase |
| Pharmacology | |
|
ATC code |
B05XA12 (WHO) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
|
Main hazards |
Moderately toxic, irritant[2] |
| GHS labelling: | |
|
Pictograms |
  
|
|
Signal word |
Danger |
|
Hazard statements |
H302, H314, H410 |
|
Precautionary statements |
P273, P280, P301+P330+P331, P305+P351+P338, P308+P310 |
| NFPA 704 (fire diamond) |
3 0 0 |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose) |
350 mg/kg (rat, oral) 350 mg/kg (mouse, oral) 200 mg/kg (guinea pig, oral) 1100 mg/kg (rat, oral) 1250 mg/kg (mouse, oral)[4] |
|
LC50 (median concentration) |
1260 mg/m3 (rat, 30 min) 1180 mg-min/m3[4] |
| NIOSH (US health exposure limits): | |
|
PEL (Permissible) |
TWA 1 mg/m3 (fume)[3] |
|
REL (Recommended) |
TWA 1 mg/m3 ST 2 mg/m3 (fume)[3] |
|
IDLH (Immediate danger) |
50 mg/m3 (fume)[3] |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
|
Other anions |
Zinc fluoride Zinc bromide Zinc iodide |
|
Other cations |
Cadmium chloride Mercury(II) chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water.[5] This salt is hygroscopic and even deliquescent. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.
Structure and properties[edit]
Four crystalline forms (polymorphs) of ZnCl2 are known: α, β, γ, and δ. Each case features tetrahedral Zn2+ centers.[6]
| Form | Symmetry | Pearson symbol | Group | No | a (nm) | b (nm) | c (nm) | Z | ρ (g/cm3) |
|---|---|---|---|---|---|---|---|---|---|
| α | tetragonal | tI12 | I42d | 122 | 0.5398 | 0.5398 | 0.64223 | 4 | 3.00 |
| β | tetragonal | tP6 | P42/nmc | 137 | 0.3696 | 0.3696 | 1.071 | 2 | 3.09 |
| γ | monoclinic | mP36 | P21/c | 14 | 0.654 | 1.131 | 1.23328 | 12 | 2.98 |
| δ | orthorhombic | oP12 | Pna21 | 33 | 0.6125 | 0.6443 | 0.7693 | 4 | 2.98 |
Here a, b, and c are lattice constants, Z is the number of structure units per unit cell, and ρ is the density calculated from the structure parameters.[7][8][9]
The orthorhombic form (δ) rapidly changes to one of the other forms on exposure to the atmosphere. A possible explanation is that the OH− ions originating from the absorbed water facilitate the rearrangement.[6] Rapid cooling of molten ZnCl2 gives a glass.[10]
Molten ZnCl2 has a high viscosity at its melting point and a comparatively low electrical conductivity, which increases markedly with temperature.[11][12] As indicated by a Raman scattering study, the viscosity is explained by the presence of polymers,[13]. Neutron scattering study indicated the presence of tetrahedral {ZnCl4} centers, which requires aggregation of ZnCl2 monomers as well..[14]
In the gas phase, ZnCl2 molecules are linear with a bond length of 205 pm.
Hydrates[edit]
Five hydrates of zinc chloride are known: ZnCl2(H2O)n with n = 1, 1.5, 2.5, 3 and 4.[15] The tetrahydrate ZnCl2(H2O)4 crystallizes from aqueous solutions of zinc chloride.[15]
Preparation and purification[edit]
Anhydrous ZnCl2 can be prepared from zinc and hydrogen chloride:
- Zn + 2 HCl → ZnCl2 + H2
Hydrated forms and aqueous solutions may be readily prepared similarly by treating Zn metal, zinc carbonate, zinc oxide, and zinc sulfide with hydrochloric acid:
- ZnS + 2 HCl + 4 H2O → ZnCl2(H2O)4 + H2S
Unlike many other elements, zinc essentially exists in only one oxidation state, 2+, which simplifies the purification of the chloride.
Commercial samples of zinc chloride typically contain water and products from hydrolysis as impurities. Such samples may be purified by recrystallization from hot dioxane. Anhydrous samples can be purified by sublimation in a stream of hydrogen chloride gas, followed by heating the sublimate to 400 °C in a stream of dry nitrogen gas.[16] Finally, the simplest method relies on treating the zinc chloride with thionyl chloride.[17]
Reactions[edit]
Molten anhydrous ZnCl2 at 500–700 °C dissolves zinc metal, and, on rapid cooling of the melt, a yellow diamagnetic glass is formed, which Raman studies indicate contains the Zn2+
2 ion.[15]
A number of salts containing the tetrachlorozincate anion, ZnCl2−
4, are known.[11] «Caulton’s reagent», V2Cl3(thf)6Zn2Cl6 is an example of a salt containing Zn2Cl2−
6.[18][19]
The compound Cs3ZnCl5 contains tetrahedral ZnCl2−
4 and Cl− anions.[6] No compounds containing the ZnCl4−
6 ion have been characterized.[6]
Zinc chloride dissolves readily in water to give ZnClxH2O(4−x) species and some free chloride.[20][21][22] Aqueous solutions of ZnCl2 are acidic: a 6 M aqueous solution has a pH of 1.[15] The acidity of aqueous ZnCl2 solutions relative to solutions of other Zn2+ salts (say the sulfate) is due to the formation of the tetrahedral chloro aqua complexes where the reduction in coordination number from 6 to 4 further reduces the strength of the O–H bonds in the solvated water molecules.[23]
In alkali solution, zinc chloride converts to various zinc hydroxychlorides. These include Zn(OH)3Cl2−, Zn(OH)2Cl2−
2, ZnOHCl2−
3, and the insoluble Zn5(OH)8Cl2·H2O. The latter is the mineral simonkolleite.[24] When zinc chloride hydrates are heated, HCl gas evolves and hydroxychlorides result.[25]
When solutions of zinc chloride are treated with ammonia, various complexes of «ammines» are produced. These include Zn(NH3)4Cl2·H2O and on concentration ZnCl2(NH3)2.[26] The former contains the Zn(NH3)62+ ion,[6] and the latter is molecular with a distorted tetrahedral geometry.[27] The species in aqueous solution have been investigated and show that Zn(NH3)42+ is the main species present with Zn(NH3)3Cl+ also present at lower NH3:Zn ratio.[28]
Aqueous zinc chloride reacts with zinc oxide to form an amorphous cement that was first investigated in 1855 by Stanislas Sorel. Sorel later went on to investigate the related magnesium oxychloride cement, which bears his name.[29]
When hydrated zinc chloride is heated, one obtains a residue of Zn(OH)Cl e.g.[30]
- ZnCl2·2H2O → ZnCl(OH) + HCl + H2O
The compound ZnCl2·1⁄2HCl·H2O may be prepared by careful precipitation from a solution of ZnCl2 acidified with HCl. It contains a polymeric anion (Zn2Cl5−)n with balancing monohydrated hydronium ions, H5O2+ ions.[6][31]
Cellulose dissolves in aqueous solutions of ZnCl2, and zinc-cellulose complexes have been detected.[32] Cellulose also dissolves in molten ZnCl2 hydrate and carboxylation and acetylation performed on the cellulose polymer.[33]
Thus, although many zinc salts have different formulas and different crystal structures, these salts behave very similarly in aqueous solution. For example, solutions prepared from any of the polymorphs of ZnCl2, as well as other halides (bromide, iodide), and the sulfate can often be used interchangeably for the preparation of other zinc compounds. Illustrative is the preparation of zinc carbonate:
- ZnCl2(aq) + Na2CO3(aq) → ZnCO3(s) + 2 NaCl(aq)
Role in organic chemistry[edit]
Zinc chloride is used as a catalyst or reagent in diverse reactions conducted on an industrial scale. The partial hydrolysis of benzal chloride in the presence of zinc chloride is the main route to benzoyl chloride. It serves as a catalyst for the production of methylene-bis(dithiocarbamate).[5]
The combination of hydrochloric acid and ZnCl2, known as the «Lucas reagent», is effective for the preparation of alkyl chlorides from alcohols. Similar reactions are the basis of industrial routes from methanol and ethanol respectively to methyl chloride and ethyl chloride.
Laboratory syntheses[edit]
Zinc chloride is a common reagent in the laboratory useful Lewis acid in organic chemistry.[34]
Molten zinc chloride catalyses the conversion of methanol to hexamethylbenzene:[35]
- 15 CH
3OH → C
6(CH
3)
6 + 3 CH
4 + 15 H
2O
Other examples include catalyzing (A) the Fischer indole synthesis,[36] and also (B) Friedel-Crafts acylation reactions involving activated aromatic rings[37][38]
Related to the latter is the classical preparation of the dye fluorescein from phthalic anhydride and resorcinol, which involves a Friedel-Crafts acylation.[39] This transformation has in fact been accomplished using even the hydrated ZnCl2 sample shown in the picture above.
Zinc chloride also activates benzylic and allylic halides towards substitution by weak nucleophiles such as alkenes:[40]
In similar fashion, ZnCl2 promotes selective NaBH3CN reduction of tertiary, allylic or benzylic halides to the corresponding hydrocarbons.
Zinc chloride is also a useful starting reagent for the synthesis of many organozinc reagents, such as those used in the palladium catalyzed Negishi coupling with aryl halides or vinyl halides.[41] In such cases the organozinc compound is usually prepared by transmetallation from an organolithium or a Grignard reagent, for example:
Zinc enolates, prepared from alkali metal enolates and ZnCl2, provide control of stereochemistry in aldol condensation reactions due to chelation on to the zinc. In the example shown below, the threo product was favored over the erythro by a factor of 5:1 when ZnCl2 in DME/ether was used.[42] The chelate is more stable when the bulky phenyl group is pseudo-equatorial rather than pseudo-axial, i.e., threo rather than erythro.
Other uses[edit]
As a metallurgical flux[edit]
The use of zinc chloride as a flux, sometimes in a mixture with ammonium chloride (see also Zinc ammonium chloride), involves the production of HCl and its subsequent reaction with surface oxides.
Zinc chloride reacts with metal oxides (MO) to give derivatives of the idealized formula MZnOCl2.[43][additional citation(s) needed] This reaction is relevant to the utility of ZnCl2 solution as a flux for soldering — it dissolves passivating oxides, exposing the clean metal surface.[43] Fluxes with ZnCl2 as an active ingredient are sometimes called «tinner’s fluid».
Zinc chloride forms two salts with ammonium chloride: (NH4)2ZnCl4 and (NH4)3ClZnCl4, which decompose on heating liberating HCl, just as zinc chloride hydrate does. The action of zinc chloride/ammonium chloride fluxes, for example, in the hot-dip galvanizing process produces H2 gas and ammonia fumes.[44]
In textile and paper processing[edit]
Concentrated aqueous solutions of zinc chloride (more than 64% weight/weight zinc chloride in water) have dissolving starch, silk, and cellulose.
Relevant to its affinity for these materials, ZnCl2 is used as a fireproofing agent and in fabric «refresheners» such as Febreze. Vulcanized fibre is made by soaking paper in concentrated zinc chloride.
Smoke grenades[edit]
The zinc chloride smoke mixture («HC») used in smoke grenades contains zinc oxide, hexachloroethane and granular aluminium powder, which, when ignited, react to form zinc chloride, carbon and aluminium oxide smoke, an effective smoke screen.[45]
Fingerprint detection[edit]
Ninhydrin reacts with amino acids and amines to form a colored compound «Ruhemann’s purple» (RP). Spraying with a zinc chloride solution forms a 1:1 complex RP:ZnCl(H2O)2, which is more readily detected as it fluoresces more intensely than RP.[46]
Disinfectant and wood preservative[edit]
Dilute aqueous zinc chloride was used as a disinfectant under the name «Burnett’s Disinfecting Fluid».
[47] From 1839 Sir William Burnett promoted its use as a disinfectant as well as a wood preservative.[48] The Royal Navy conducted trials into its use as a disinfectant in the late 1840s, including during the cholera epidemic of 1849; and at the same time experiments were conducted into its preservative properties as applicable to the shipbuilding and railway industries. Burnett had some commercial success with his eponymous fluid. Following his death however, its use was largely superseded by that of carbolic acid and other proprietary products.
Safety[edit]
Zinc chloride is a chemical irritant of the eyes, skin, and respiratory system.[5][49]
Additional reading[edit]
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- The Merck Index, 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960.
- D. Nicholls, Complexes and First-Row Transition Elements, Macmillan Press, London, 1973.
- J. March, Advanced Organic Chemistry, 4th ed., p. 723, Wiley, New York, 1992.
- G. J. McGarvey, in Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation, (R. M. Coates, S. E. Denmark, eds.), pp. 220–3, Wiley, New York, 1999.
References[edit]
- ^ a b O’Neil, M. J.; et al. (2001). The Merck index : an encyclopedia of chemicals, drugs, and biologicals. N. J.: Whitehouse Station. ISBN 978-0911910131.
- ^ Zinc chloride toxicity
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. «#0674». National Institute for Occupational Safety and Health (NIOSH).
- ^ a b «Zinc chloride fume». Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c Dieter M. M. Rohe; Hans Uwe Wolf (2007). «Zinc Compounds». Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–6. doi:10.1002/14356007.a28_537.
- ^ a b c d e f Wells, A. F. (1984). Structural Inorganic Chemistry. Oxford: Clarendon Press. ISBN 978-0-19-855370-0.
- ^ Oswald, H. R.; Jaggi, H. (1960). «Zur Struktur der wasserfreien Zinkhalogenide I. Die wasserfreien Zinkchloride». Helvetica Chimica Acta. 43 (1): 72–77. doi:10.1002/hlca.19600430109.
- ^ Brynestad, J.; Yakel, H. L. (1978). «Preparation and Structure of Anhydrous Zinc Chloride». Inorganic Chemistry. 17 (5): 1376–1377. doi:10.1021/ic50183a059.
- ^ Brehler, B. (1961). «Kristallstrukturuntersuchungen an ZnCl2«. Zeitschrift für Kristallographie. 115 (5–6): 373–402. Bibcode:1961ZK….115..373B. doi:10.1524/zkri.1961.115.5-6.373.
- ^ Mackenzie, J. D.; Murphy, W. K. (1960). «Structure of Glass-Forming Halides. II. Liquid Zinc Chloride». The Journal of Chemical Physics. 33 (2): 366–369. Bibcode:1960JChPh..33..366M. doi:10.1063/1.1731151.
- ^ a b Prince, R. H. (1994). King, R. B. (ed.). Encyclopedia of Inorganic Chemistry. John Wiley & Sons. ISBN 978-0-471-93620-6.
- ^ Ray, H. S. (2006). Introduction to Melts: Molten Salts, Slags and Glasses. Allied Publishers. ISBN 978-81-7764-875-1.
- ^ Danek, V. (2006). Physico-Chemical Analysis of Molten Electrolytes. Elsevier. ISBN 978-0-444-52116-3.
- ^ Price, D. L.; Saboungi, M.-L.; Susman, S.; Volin, K. J.; Wright, A. C. (1991). «Neutron Scattering Function of Vitreous and Molten Zinc Chloride». Journal of Physics: Condensed Matter. 3 (49): 9835–9842. Bibcode:1991JPCM….3.9835P. doi:10.1088/0953-8984/3/49/001. S2CID 250902741.
- ^ a b c d Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- ^ Glenn J. McGarvey Jean-François Poisson Sylvain Taillemaud (2016). «Zinc chloride». Encyclopedia of Reagents for Organic Synthesis: 1–20. doi:10.1002/047084289X.rz007.pub3. ISBN 9780470842898.
- ^ Pray, A. P. (1990). Anhydrous Metal Chlorides. Inorganic Syntheses. Vol. 28. pp. 321–322.
- ^ Mulzer, J.; Waldmann, H., eds. (1998). Organic Synthesis Highlights. Vol. 3. Wiley-VCH. ISBN 978-3-527-29500-5.
- ^ Bouma, R. J.; Teuben, J. H.; Beukema, W. R.; Bansemer, R. L.; Huffman, J. C.; Caulton, K. G. (1984). «Identification of the Zinc Reduction Product of VCl3 · 3THF as [V2Cl3(THF)6]2[Zn2Cl6]». Inorganic Chemistry. 23 (17): 2715–2718. doi:10.1021/ic00185a033.
- ^ Irish, D. E.; McCarroll, B.; Young, T. F. (1963). «Raman Study of Zinc Chloride Solutions». The Journal of Chemical Physics. 39 (12): 3436–3444. Bibcode:1963JChPh..39.3436I. doi:10.1063/1.1734212.
- ^ Yamaguchi, T.; Hayashi, S.; Ohtaki, H. (1989). «X-Ray Diffraction and Raman Studies of Zinc(II) Chloride Hydrate Melts, ZnCl2 · R H2O (R = 1.8, 2.5, 3.0, 4.0, and 6.2)». The Journal of Physical Chemistry. 93 (6): 2620–2625. doi:10.1021/j100343a074.
- ^ Pye, C. C.; Corbeil, C. R.; Rudolph, W. W. (2006). «An ab initio Investigation of Zinc Chloro Complexes». Physical Chemistry Chemical Physics. 8 (46): 5428–5436. Bibcode:2006PCCP….8.5428P. doi:10.1039/b610084h. ISSN 1463-9076. PMID 17119651. S2CID 37521287.
- ^ Brown, I. D. (2006). The Chemical Bond in Inorganic Chemistry: The Bond Valence Model. Oxford University Press. ISBN 978-0-19-929881-5.
- ^ Zhang, X. G. (1996). Corrosion and Electrochemistry of Zinc. Springer. ISBN 978-0-306-45334-2. Staff writer(s). «Simonkolleite Mineral Data». webmineral.com. Retrieved October 16, 2014.
- ^ Feigl, F.; Caldas, A. (1956). «Some Applications of Fusion Reactions with Zinc Chloride in Inorganic Spot Test Analysis». Microchimica Acta. 44 (7–8): 1310–1316. doi:10.1007/BF01257465. S2CID 96823985.
- ^ Vulte, H. T. (2007). Laboratory Manual of Inorganic Preparations. Read Books. ISBN 978-1-4086-0840-1.
- ^ Yamaguchi, T.; Lindqvist, O. (1981). «The Crystal Structure of Diamminedichlorozinc(II), ZnCl2(NH3)2. A New Refinement» (PDF). Acta Chemica Scandinavica A. 35 (9): 727–728. doi:10.3891/acta.chem.scand.35a-0727.
- ^ Yamaguchi, T.; Ohtaki, H. (1978). «X-Ray Diffraction Studies on the Structures of Tetraammine- and Triamminemonochlorozinc(II) Ions in Aqueous Solution». Bulletin of the Chemical Society of Japan. 51 (11): 3227–3231. doi:10.1246/bcsj.51.3227.
- ^ Wilson, A. D.; Nicholson, J. W. (1993). Acid-Base Cements: Their Biomedical and Industrial Applications. Cambridge University Press. ISBN 978-0-521-37222-0.
- ^ House, J. E. (2008). Inorganic Chemistry. Academic Press. ISBN 978-0-12-356786-4.
- ^ Mellow, J. W. (1946). A Comprehensive Treatise on Inorganic and Theoretical Chemistry. Longmans, Green.
- ^ Xu, Q.; Chen, L.-F. (1999). «Ultraviolet Spectra and Structure of Zinc-Cellulose Complexes in Zinc Chloride Solution». Journal of Applied Polymer Science. 71 (9): 1441–1446. doi:10.1002/(SICI)1097-4628(19990228)71:9<1441::AID-APP8>3.0.CO;2-G.
- ^ Fischer, S.; Leipner, H.; Thümmler, K.; Brendler, E.; Peters, J. (2003). «Inorganic Molten Salts as Solvents for Cellulose». Cellulose. 10 (3): 227–236. doi:10.1023/A:1025128028462. S2CID 92194004.
- ^ Olah, George A.; Doggweiler, Hans; Felberg, Jeff D.; Frohlich, Stephan; Grdina, Mary Jo; Karpeles, Richard; Keumi, Takashi; Inaba, Shin-ichi; Ip, Wai M.; Lammertsma, Koop; Salem, George; Tabor, Derrick (1984). «Onium Ylide chemistry. 1. Bifunctional acid-base-catalyzed conversion of heterosubstituted methanes into ethylene and derived hydrocarbons. The onium ylide mechanism of the C1 → C2 conversion». J. Am. Chem. Soc. 106 (7): 2143–2149. doi:10.1021/ja00319a039.
- ^ Chang, Clarence D. (1983). «Hydrocarbons from Methanol». Catal. Rev. — Sci. Eng. 25 (1): 1–118. doi:10.1080/01614948308078874.
- ^ Shriner, R. L.; Ashley, W. C.; Welch, E. (1942). «2-Phenylindole». Organic Syntheses. 22: 98. doi:10.15227/orgsyn.022.00981955.; Collective Volume, vol. 3, p. 725
- ^ Cooper, S. R. (1941). «Resacetophenone». Organic Syntheses. 21: 103. doi:10.15227/orgsyn.021.0103.; Collective Volume, vol. 3, p. 761
- ^ Dike, S. Y.; Merchant, J. R.; Sapre, N. Y. (1991). «A New and Efficient General Method for the Synthesis of 2-Spirobenzopyrans: First Synthesis of Cyclic Analogues of Precocene I and Related Compounds». Tetrahedron. 47 (26): 4775–4786. doi:10.1016/S0040-4020(01)86481-4.
- ^ Furnell, B. S. (1989). Vogel’s Textbook of Practical Organic Chemistry (5th ed.). New York: Longman/Wiley.
- ^ Bauml, E.; Tschemschlok, K.; Pock, R.; Mayr, H. (1988). «Synthesis of γ-Lactones from Alkenes Employing p-Methoxybenzyl Chloride as +CH2-CO2− Equivalent» (PDF). Tetrahedron Letters. 29 (52): 6925–6926. doi:10.1016/S0040-4039(00)88476-2.
- ^ Kim, S.; Kim, Y. J.; Ahn, K. H. (1983). «Selective Reduction of Tertiary, Allyl, and Benzyl Halides by Zinc-Modified Cyanoborohydride in Diethyl Ether». Tetrahedron Letters. 24 (32): 3369–3372. doi:10.1016/S0040-4039(00)86272-3.
- ^ House, H. O.; Crumrine, D. S.; Teranishi, A. Y.; Olmstead, H. D. (1973). «Chemistry of Carbanions. XXIII. Use of Metal Complexes to Control the Aldol Condensation». Journal of the American Chemical Society. 95 (10): 3310–3324. doi:10.1021/ja00791a039.
- ^ a b Wiberg, Nils (2007). Lehrbuch der Anorganischen Chemie [Holleman & Wiberg, Textbook of Inorganic chemistry] (in German). de Gruyter, Berlin. p. 1491. ISBN 978-3-11-017770-1.
- ^ American Society for Metals (1990). ASM handbook. ASM International. ISBN 978-0-87170-021-6.
- ^ Sample, B. E. (1997). Methods for Field Studies of Effects of Military Smokes, Obscurants, and Riot-control Agents on Threatened and Endangered Species. DIANE Publishing. ISBN 978-1-4289-1233-5.
- ^ Menzel, E. R. (1999). Fingerprint Detection with Lasers. CRC Press. ISBN 978-0-8247-1974-6.
- ^ Watts, H. (1869). A Dictionary of Chemistry and the Allied Branches of Other Sciences. Longmans, Green.
- ^ McLean, David (April 2010). «Protecting wood and killing germs: ‘Burnett’s Liquid’ and the origins of the preservative and disinfectant industries in early Victorian Britain». Business History. 52 (2): 285–305. doi:10.1080/00076791003610691. S2CID 154790730.
- ^ «NIOSH Pocket Guide to Chemical Hazards». CDC.gov. Retrieved 30 October 2020.
External links[edit]
- Grades and Applications of Zinc Chloride
- PubChem ZnCl2 summary.
- хлорид цинка
- zink chloride
Большой англо-русский и русско-английский словарь.
2001.
Смотреть что такое «хлорид цинка» в других словарях:
-
Хлорид цинка — (хлористый цинк, дихлорид цинка, паяльная кислота) химическое соединение цинка с хлором, имеющее формулу ZnCl2 … Википедия
-
хлорид цинка — хлористый цинк … Cловарь химических синонимов I
-
Цинка хлорид — Хлорид цинка Хлорид цинка (хлористый цинк) химическое соединение цинка с хлором, имеющее формулу ZnCl2. Белые гигроскопичные кристаллы. Содержание 1 Свойства … Википедия
-
Цинка галогениды — Существуют: Фторид цинка Хлорид цинка Бромид цинка Иодид цинка … Википедия
-
ЦИНКА СЕМЕЙСТВО — ПОДГРУППА IIB. СЕМЕЙСТВО ЦИНКА ЦИНК, КАДМИЙ, РТУТЬ Положение элементов семейства цинка как членов рядов переходных металлов, рассмотрено ранее (см. разд. Подгруппа IB и Переходные элементы). Хотя валентный электрон, отличающий их от элементов… … Энциклопедия Кольера
-
Хлорид вольфрама(VI) — Общие … Википедия
-
Хлорид вольфрама(II) — Общие Систематическое наименование Хлорид вольфрама(II) Традиционные названия Хлористый вольфрам; гексамер дихлорида вольфрама Химическая формула WCl2 Физические свойства … Википедия
-
Хлорид вольфрама(IV) — Общие Систематическое наименование Хлорид вольфрама(IV) Традиционные названия хлористый вольфрам Химическая формула WCl4 Физические свойства Сос … Википедия
-
Хлорид вольфрама(V) — Общие Систематическое наименование Хлорид вольфрама(V) Традиционные названия Хлористый вольфрам Химическая формула WCl5 Физические свойства Состо … Википедия
-
Хлорид ванадия(II) — Раствор хлорида ванадия(II) Общие Систематическое наименование Хлорид Ванадия(II) … Википедия
-
Хлорид водорода (соляная кислота) — Хлорид водорода (HCl) это бесцветный дымящийся газ с удушливым запахом, получаемым действием водорода (или воды и кокса) на хлор или действием серной кислоты на хлорид натрия. Он легко сжижается под давлением и легко растворим в воде. Хранится… … Официальная терминология
Хлорид цинка представляет собой химическое соединение двух элементов — хлора и цинка — и обозначается формулой ZnCl2. Данное вещество представляет собой белые кристаллы.
Хлорид цинка достаточно легко растворяется в воде — при комнатной температуре его растворимость равна 80%. Плавится хлорид при 322°C, а закипает при 722°C.
Получается хлорид цинка двумя путями. Первый вариант: цинк или его окись растворяется в соляной кислоте, после чего из полученной смеси выпариваются растворы. Второй вариант: цинк (в жидком состоянии) нагревается в токе хлора.
Гидролиз хлорида цинка происходит по катиону и имеет следующую формулу: ZnCl2 + H2O = ZnOHCl + HCl. Среда получившегося раствора — кислотная.
Промышленным производством хлорид цинка выпускается в двух формах: твердой и жидкой. В твердой форме вещество должно иметь вид белых чешуек, допустимо незначительное окрашивание в какой-либо цвет. В виде раствора хлорид должен быть бесцветным или иметь слабый желтоватый оттенок. Раствор может быть слегка замутнен.
В твердой форме массовая доля хлорида не должна быть меньше 97,7%, в растворе — 50%. Цинк хлористый не горюч.
Вещество весьма опасно для окружающей природы и человека: твердое вещество имеет 2 степень токсичности. Вещество, при попадании на кожу и слизистые оболочки человека или животного, вызывает раздражение, при более длительном контакте с кожным покровом вызывает ожоги, разъедает ткани. Образующиеся таким способ раны очень трудно заживают.
Опасность представляет и попадание вещества в дыхательные пути. В малых дозах вызывает першение в носоглотке и горле, сухой кашель. При вдыхании большого количества хлорида возможно возникновение одышки и так называемого клокочущего дыхания.
При попадании вещества на слизистую глаз, пострадавший испытывает достаточно интенсивную режущую боль. Если глаза не промыть сразу же, возможно возникновение полной или частичной слепоты.
Ввиду токсичности цинка хлористого, при его транспортировке и использовании необходимо соблюдать повышенную осторожность. Кристаллический хлорид цинка упаковывают в мешки или барабаны из углеродистой стали, раствор транспортируется в стальных бочках или специальных цистернах.
Транспортировка вещества осуществляется как железнодорожным, так и автомобильным, и морским транспортом. Вещество перевозится только в крытых отсеках и все время транспортировки ответственное лицо обязано следить за целостностью упаковки.
При работе с цинком хлористым рабочие обязаны одевать соответствующую уровню концентрации вещества в воздухе спецодежду, прорезиненные перчатки, защитные очки и респираторы.
Ни в коем случае нельзя допускать попадания хлористого цинка в водоемы и системы канализации.
Цинк хлористый применяется во многих сферах производства. Его используют для пропитывания деревянных деталей с целью дезинфекции (например, деревянные шпалы). Это вещество участвует в изготовлении фибры, многих красителей, многих зубных цементов, хлопка, цианида цинка, алюминия и даже ванилина. Кроме того, цинк хлористый используют для очистки металлических поверхностей перед покраской, пайкой и хромированием. Немалую роль это вещество играет и при очистке нефти, анализе проб угля и изготовлении гальванических батарей.
Хлорид цинка также используется для окраски ситца, получения волокон вискозы, в качестве электролита — при оцинковании. Возможно использование вещества в медицине — в качестве препарата, останавливающего гниение; в садоводстве — в качестве микроудобрения.
Помимо этого, хлорид цинка, благодаря своей блестящей способности жадно впитывать влагу из воздуха, используется в качестве осушителя. Также незаменим он и в пожарном деле, так как участвует в изготовлении огнестойкой пены и пропитке тканей и картона.
Текущая версия страницы пока не проверялась опытными участниками и может значительно отличаться от версии, проверенной 12 апреля 2022 года; проверки требуют 3 правки.
Водный раствор хлорида цинка, также известный, как «Паяльная кислота»
Хлори́д ци́нка (хло́ристый цинк, дихлори́д цинка) — химическое соединение цинка с хлором, имеющее формулу ZnCl2.
Представляет собой белые, очень гигроскопичные кристаллы.
Свойства[править | править код]
Физические свойства[править | править код]
- Молекулярная масса: 136,2954.
- Температура плавления: 318 °C.
- Температура кипения: 732 °C.
- Растворимость в воде при 20 °C: 79,8 %.
Химические свойства[править | править код]
Концентрированные растворы имеют кислую среду, так как в результате гидролиза в воде присутствуют ионы .
Получение[править | править код]
- растворение цинка или его оксида в соляной кислоте с последующим выпариванием раствора;
- нагревание расплавленного цинка в потоке хлора.
Применение[править | править код]
- ситцепечатание;
- изготовление зубных цементов;
- антисептическая пропитка дерева (например, шпал);
- очистка поверхности металлов от оксидов перед пайкой (известен как «Паяльная кислота»);
- компонент при производстве фибры;
- рафинирование расплавленных цинковых сплавов;
- фракционный анализ угольных проб;
- в гальванических элементах.
Токсичность[править | править код]
Хлорид цинка токсичен, ирритант. При попадании на кожу вызывает химические ожоги. Особенно опасно попадание в глаза.
После попадания на кожу необходимо немедленно удалить вещество с использованием мыла и большого количества воды. При попадании в глаза промыть большим количеством воды, использовать глазные капли.
Минимальная смертельная доза (ЛД50) — 200 мг/кг. Смертельная доза для человека орально — 3—5 г.
Примечания[править | править код]
Литература[править | править код]
- Гончаров А. И., Корнилов М. Ю. Справочник по химии. — Киев: «Вища школа», 1977.
- статья «Цинка хлорид» в БСЭ
- ГОСТ 4529-78 Цинк хлористый.
Zinc is a chemical element with the symbol Zn. Its atomic number is 30, and its electron configuration is [Ar]3d10 4s2. It is mostly used for galvanizing other metals in order to protect them from rusting. It is a slightly brittle metal at room temperature and has a silvery-greyish appearance when oxidation is removed. It occurs abundantly in minerals, and it is an essential micronutrient for both plants and animals. It is an essential trace element in the human body, where it is found in high concentrations of red blood cells. Chlorine is a chemical element with symbol Cl .it’s atomic number is 17 and it’s electron configuration is [Ne]3s2 3p5 or 1s2 2s2 2p6 3s2 3p5.It is the second lightest member of the halogen elements of the periodic table. It is a yellow-green gas at room temperature. It is used especially as a bleach, oxidizing agent, and disinfectant in water purification. Chlorine was first isolated in 1774 by the Swiss-German chemist Carl Wilhelm Scheele.
Zinc Chloride Formula
Zinc Chloride is a chemical compound of zinc and chlorine with the formula ZnCl2. It is also called Zinc Dichloride, butter of zinc, or zinc(II)chloride. It is a colorless liquid and highly soluble in water, and it exhibits hygroscopic qualities. It is used in dry cells as an electrolyte. It is mildly corrosive to metals. It causes burns to the eyes, skin, and mucous membranes. There are nine different crystalline forms of ZnCl2 that are currently known .this chemical compound is strongly deliquescent and is utilized as a drying agent and as a flux. It can be prepared by a direct reaction or by evaporating the aqueous solution formed in various reactions.
Preparation Of Zinc Chloride
Anhydrous Zinc Chloride can be prepared from zinc and hydrogen chloride. Dissolve your zinc in dilute hydrochloric acid. It will give off hydrogen, and the remaining will contain Zinc Chloride.
Zn + 2 HCl ⇢ ZnCl2 + H
In order to obtain a hydrated form of the compound hydrochloric acid can be used to treat zinc instead of hydrogen chloride. Hydrochloric acid also reacts with zinc sulfide to form Zinc Chloride and also produces hydrogen sulfide.
Zn + 2 HCL ⇢ ZnCl2 + H2S
Structure Of Zinc Chloride
The zinc Chloride chemical formula is ZnCl2. It is an inorganic binary salt. A molecule of Zinc Chloride features ionic bonding between the zinc cation (Zn2+) and the chloride anions (Cl–). Then the structure of Zinc Chloride is shown below.
Physical Properties Of Zinc Chloride
- Zinc Chloride molecular weight is 136.315 gr/mole
- Its density is 2.907 g/cm3
- Its boiling point is 732°C
- Its melting point is 290°C
Chemical Properties Of Zinc Chloride
- Zinc Chloride chemical formula is ZnCl2
- It is a white crystalline solid
- It is very soluble in water, ether, alcohol, and glycerol
- it should be safeguarded from all sources of moisture
- With excess water, zinc oxychlorides are formed. When Zinc Chloride is dissolved in water, the solution becomes acidic.
- When heated ZnCl2, the hydrated form of zinc chloride loses water, and small quantities of ZnCl(OH) are obtained.
Uses Of Zinc Chloride
- Zinc Chloride is used in dry cells as an electrolyte.
- It is used in the manufacture of magnesia cement for dental fillings.
- It can be used as a catalyst in organic processes.
- It is used in certain mouthwashes as an active ingredient.
- It is used in the manufacturing of various dyes, intermediate chemicals, and solvents.
- It is a primary ingredient in smoke bombs used by the military for screening purposes.
- It is a medication used to treat zinc deficiencies and is also used as an alternative medicine for the cause of dead tissue to cure skin cancers.
Sample Questions
Question 1: What are the hazards and handling considerations of Zinc Chloride?
Answer:
Toxic Zinc Chloride is corrosive by ingestion and highly irritant by inhalation. Zinc Chloride causes ulceration and burns, and chronic exposure has been associated with anorexia, fatigue, and weight loss. Prior to working with Zinc Chloride, you should be trained on its proper handling and storage. To handle It, minimize dust generation and accumulation. Do not get in eyes on skin or on clothing. Do not ingest or inhale. Use only in a chemical fume hood.
Question 2: What is Zinc Chloride Battery?
Answer:
Zinc Chloride cells use a higher concentration of anolyte, which is primarily composed of zinc chloride which can produce a more consistent voltage output in high drain applications
Question 3: Is Zinc Chloride harmful to the environment?
Answer:
The substance is very toxic to aquatic organisms. Zinc Chloride is a severe marine pollutant that may cause long-term adverse effects on the aquatic environment. It is strongly advised not to let the chemical enter the environment.
Question 4: Is Zinc Chloride safe in mouthwash?
Answer:
Yes. Zinc Chloride has a long safe history of use as an effective mouthwash ingredient. Zinc is formulated into oral health products to control plaque, reduce malodor and inhibit calculus formation. Many experts believe the addition of zinc ions in mouthwash is the best way to help reduce bacteria.
Question 5: What chemicals react with zinc?
Answer:
Zinc is a chemical element with the symbol Zn. Its atomic number is 30. It reacts with acids, alkalis, and other non-metals. Extremely pure zinc reacts only slowly at room temperature with acids Strong acids such as hydrochloric or sulfuric acid can remove the passivating layer, and the subsequent reaction with the acid releases hydrogen gas.
Question 6: Why zinc is used in dry cells?
Answer:
Zinc is a medium reactive metal and is oxidized slowly by air .it is electropositive as compared to carbon which is used as a positive electrode in a dry cell. So it donates electrons, and a current is produced. It is not very reactive and is consumed slowly so it is used in dry cells.
Zinc is a chemical element with the symbol Zn. Its atomic number is 30, and its electron configuration is [Ar]3d10 4s2. It is mostly used for galvanizing other metals in order to protect them from rusting. It is a slightly brittle metal at room temperature and has a silvery-greyish appearance when oxidation is removed. It occurs abundantly in minerals, and it is an essential micronutrient for both plants and animals. It is an essential trace element in the human body, where it is found in high concentrations of red blood cells. Chlorine is a chemical element with symbol Cl .it’s atomic number is 17 and it’s electron configuration is [Ne]3s2 3p5 or 1s2 2s2 2p6 3s2 3p5.It is the second lightest member of the halogen elements of the periodic table. It is a yellow-green gas at room temperature. It is used especially as a bleach, oxidizing agent, and disinfectant in water purification. Chlorine was first isolated in 1774 by the Swiss-German chemist Carl Wilhelm Scheele.
Zinc Chloride Formula
Zinc Chloride is a chemical compound of zinc and chlorine with the formula ZnCl2. It is also called Zinc Dichloride, butter of zinc, or zinc(II)chloride. It is a colorless liquid and highly soluble in water, and it exhibits hygroscopic qualities. It is used in dry cells as an electrolyte. It is mildly corrosive to metals. It causes burns to the eyes, skin, and mucous membranes. There are nine different crystalline forms of ZnCl2 that are currently known .this chemical compound is strongly deliquescent and is utilized as a drying agent and as a flux. It can be prepared by a direct reaction or by evaporating the aqueous solution formed in various reactions.
Preparation Of Zinc Chloride
Anhydrous Zinc Chloride can be prepared from zinc and hydrogen chloride. Dissolve your zinc in dilute hydrochloric acid. It will give off hydrogen, and the remaining will contain Zinc Chloride.
Zn + 2 HCl ⇢ ZnCl2 + H
In order to obtain a hydrated form of the compound hydrochloric acid can be used to treat zinc instead of hydrogen chloride. Hydrochloric acid also reacts with zinc sulfide to form Zinc Chloride and also produces hydrogen sulfide.
Zn + 2 HCL ⇢ ZnCl2 + H2S
Structure Of Zinc Chloride
The zinc Chloride chemical formula is ZnCl2. It is an inorganic binary salt. A molecule of Zinc Chloride features ionic bonding between the zinc cation (Zn2+) and the chloride anions (Cl–). Then the structure of Zinc Chloride is shown below.
Physical Properties Of Zinc Chloride
- Zinc Chloride molecular weight is 136.315 gr/mole
- Its density is 2.907 g/cm3
- Its boiling point is 732°C
- Its melting point is 290°C
Chemical Properties Of Zinc Chloride
- Zinc Chloride chemical formula is ZnCl2
- It is a white crystalline solid
- It is very soluble in water, ether, alcohol, and glycerol
- it should be safeguarded from all sources of moisture
- With excess water, zinc oxychlorides are formed. When Zinc Chloride is dissolved in water, the solution becomes acidic.
- When heated ZnCl2, the hydrated form of zinc chloride loses water, and small quantities of ZnCl(OH) are obtained.
Uses Of Zinc Chloride
- Zinc Chloride is used in dry cells as an electrolyte.
- It is used in the manufacture of magnesia cement for dental fillings.
- It can be used as a catalyst in organic processes.
- It is used in certain mouthwashes as an active ingredient.
- It is used in the manufacturing of various dyes, intermediate chemicals, and solvents.
- It is a primary ingredient in smoke bombs used by the military for screening purposes.
- It is a medication used to treat zinc deficiencies and is also used as an alternative medicine for the cause of dead tissue to cure skin cancers.
Sample Questions
Question 1: What are the hazards and handling considerations of Zinc Chloride?
Answer:
Toxic Zinc Chloride is corrosive by ingestion and highly irritant by inhalation. Zinc Chloride causes ulceration and burns, and chronic exposure has been associated with anorexia, fatigue, and weight loss. Prior to working with Zinc Chloride, you should be trained on its proper handling and storage. To handle It, minimize dust generation and accumulation. Do not get in eyes on skin or on clothing. Do not ingest or inhale. Use only in a chemical fume hood.
Question 2: What is Zinc Chloride Battery?
Answer:
Zinc Chloride cells use a higher concentration of anolyte, which is primarily composed of zinc chloride which can produce a more consistent voltage output in high drain applications
Question 3: Is Zinc Chloride harmful to the environment?
Answer:
The substance is very toxic to aquatic organisms. Zinc Chloride is a severe marine pollutant that may cause long-term adverse effects on the aquatic environment. It is strongly advised not to let the chemical enter the environment.
Question 4: Is Zinc Chloride safe in mouthwash?
Answer:
Yes. Zinc Chloride has a long safe history of use as an effective mouthwash ingredient. Zinc is formulated into oral health products to control plaque, reduce malodor and inhibit calculus formation. Many experts believe the addition of zinc ions in mouthwash is the best way to help reduce bacteria.
Question 5: What chemicals react with zinc?
Answer:
Zinc is a chemical element with the symbol Zn. Its atomic number is 30. It reacts with acids, alkalis, and other non-metals. Extremely pure zinc reacts only slowly at room temperature with acids Strong acids such as hydrochloric or sulfuric acid can remove the passivating layer, and the subsequent reaction with the acid releases hydrogen gas.
Question 6: Why zinc is used in dry cells?
Answer:
Zinc is a medium reactive metal and is oxidized slowly by air .it is electropositive as compared to carbon which is used as a positive electrode in a dry cell. So it donates electrons, and a current is produced. It is not very reactive and is consumed slowly so it is used in dry cells.
Цинка хлорид
- Цинка хлорид
-
хлористый цинк, ZnCI2, белые гигроскопичные кристаллы, плотность 2,9 г/см3; tпл 322 °С; tкип 722 °С; растворимость в воде 79,8% (20 °С). Концентрированные растворы имеют кислую реакцию. Получается растворением цинка или его окиси в соляной кислоте с последующим выпариванием растворов, нагреванием жидкого цинка в токе хлора и другими методами. Применяется в ситцепечатании, для изготовления зубных цементов, для антисептической пропитки дерева, очистки поверхности металлов от окислов перед пайкой.
Лит. см. при ст. Цинк.
Большая советская энциклопедия. — М.: Советская энциклопедия.
1969—1978.
Смотреть что такое «Цинка хлорид» в других словарях:
-
Цинка хлорид — Хлорид цинка Хлорид цинка (хлористый цинк) химическое соединение цинка с хлором, имеющее формулу ZnCl2. Белые гигроскопичные кристаллы. Содержание 1 Свойства … Википедия
-
ЦИНКА ХЛОРИД — (хлористый цинк) ZnCl2, бесцветные кристаллы. Очень гигроскопичен, растворяется в воде. Применяют для пропитки дерева (напр., шпал), при травлении и пайке металлов, в химическом синтезе … Большой Энциклопедический словарь
-
цинка хлорид — ZnCl2, бесцветные кристаллы. Очень гигроскопичен, растворяется в воде. Применяют для пропитки дерева (например, шпал), при травлении и пайке металлов, в химическом синтезе. * * * ЦИНКА ХЛОРИД ЦИНКА ХЛОРИД (хлористый цинк), ZnCl2, бесцветные… … Энциклопедический словарь
-
цинка хлорид — cinko chloridas statusas T sritis chemija formulė ZnCl₂ atitikmenys: angl. zinc butter; zinc chloride; zinc dichloride rus. цинк хлористый; цинка хлорид ryšiai: sinonimas – cinko dichloridas … Chemijos terminų aiškinamasis žodynas
-
ЦИНКА ХЛОРИД — ZnCl2, бесцв., очень гигроскопичные кристаллы; известен в 3 модификациях: а тетрагон. сингонии (а = 0,540 нм, с=1,035 нм, z = 4, пространств, группа I42 моноклинной сингонии (а = 0,654 нм, b=1,131нм, с= 1,233 нм, = 90 … Химическая энциклопедия
-
ЦИНКА ХЛОРИД — ZnCl2, бесцв. кристаллы. Очень гигроскопичен, растворяется в воде. Применяют для пропитки дерева (напр., шпал), при травлении и пайке металлов, в хим. синтезе … Естествознание. Энциклопедический словарь
-
Цинкум хлоратум — Zincum chloratum, Цинка хлорид — ZnCl 2 цинка хлорид, получается взаимодействиесм соляной кислоты с цинком. Белое кристаллическое вещество, растворяется в воде. Применяется в промышленности как антисептик при обработке древесины, в бумажной промышленности, в производстве вискозы … Справочник по гомеопатии
-
Хлорид цинка — (хлористый цинк, дихлорид цинка, паяльная кислота) химическое соединение цинка с хлором, имеющее формулу ZnCl2 … Википедия
-
Цинка галогениды — Существуют: Фторид цинка Хлорид цинка Бромид цинка Иодид цинка … Википедия
-
ЦИНКА ГАЛОГЕНИДЫ — бесцв. кристаллы тетрагон. сингонии (табл.; см. также Цинка хлорид). Фторид ZnF2 кристаллизуется в структурном типе рутила; при высоких давлениях получены также моноклинная модификация со структурой типа ZrO2, кубич. типа флюорита и ромбич. со… … Химическая энциклопедия