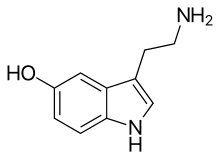
 |
|
| Clinical data | |
|---|---|
| Other names | 5-HT, 5-Hydroxytryptamine, Enteramine, Thrombocytin, 3-(β-Aminoethyl)-5-hydroxyindole, Thrombotonin |
| Physiological data | |
| Source tissues | raphe nuclei, enterochromaffin cells |
| Target tissues | system-wide |
| Receptors | 5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 |
| Agonists | Indirectly: SSRIs, MAOIs |
| Precursor | 5-HTP |
| Biosynthesis | Aromatic L-amino acid decarboxylase |
| Metabolism | MAO |
| Identifiers | |
|
IUPAC name
|
|
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| ChemSpider |
|
| KEGG |
|
| PDB ligand |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.000.054 |

|
|
| Names | |
|---|---|
| IUPAC name
5-Hydroxytryptamine |
|
| Preferred IUPAC name
3-(2-Aminoethyl)-1H-indol-5-ol |
|
| Other names
5-Hydroxytryptamine, 5-HT, Enteramine; Thrombocytin, 3-(β-Aminoethyl)-5-hydroxyindole, 3-(2-Aminoethyl)indol-5-ol, Thrombotonin |
|
| Identifiers | |
|
CAS Number |
|
|
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.000.054 |
|
IUPHAR/BPS |
|
| KEGG |
|
| MeSH | Serotonin |
|
PubChem CID |
|
| UNII |
|
|
CompTox Dashboard (EPA) |
|
|
InChI
|
|
|
SMILES
|
|
| Properties | |
|
Chemical formula |
C10H12N2O |
| Molar mass | 176.215 g/mol |
| Appearance | White powder |
| Melting point | 167.7 °C (333.9 °F; 440.8 K) 121–122 °C (ligroin)[3] |
| Boiling point | 416 ± 30 °C (at 760 Torr)[1] |
|
Solubility in water |
slightly soluble |
| Acidity (pKa) | 10.16 in water at 23.5 °C[2] |
|
Dipole moment |
2.98 D |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose) |
750 mg/kg (subcutaneous, rat),[4] 4500 mg/kg (intraperitoneal, rat),[5] 60 mg/kg (oral, rat) |
| Safety data sheet (SDS) | External MSDS |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Serotonin ([6][7][8]) or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction.[9] Approximately 90% of the serotonin that the body produces is in the intestinal tract.[10]
Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin.[11] Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is released during agitation and vasoconstriction, where it then acts as an agonist to other platelets.[12]
Approximately 90% of the human body’s total serotonin is located in the enterochromaffin cells in the GI tract, where it regulates intestinal movements.[13][14] About 8% is found in platelets and 1–2% in the CNS.[15] The serotonin is secreted luminally and basolaterally, which leads to increased serotonin uptake by circulating platelets and activation after stimulation, which gives increased stimulation of myenteric neurons and gastrointestinal motility.[16] The remainder is synthesized in serotonergic neurons of the CNS, where it has various functions. These include the regulation of mood, appetite, and sleep. Serotonin also has some cognitive functions, including memory and learning.
Several classes of antidepressants, such as the SSRIs and the SNRIs among others, interfere with the normal reabsorption of serotonin after it is done with the transmission of the signal, therefore augmenting the neurotransmitter levels in the synapses.
Serotonin secreted from the enterochromaffin cells eventually finds its way out of tissues into the blood. There, it is actively taken up by blood platelets, which store it. When the platelets bind to a clot, they release serotonin, where it can serve as a vasoconstrictor or a vasodilator while regulating hemostasis and blood clotting. In high concentrations, serotonin acts as a vasoconstrictor by contracting endothelial smooth muscle directly or by potentiating the effects of other vasoconstrictors (e.g. angiotensin II, norepinephrine). The vasoconstrictive property is mostly seen in pathologic states affecting the endothelium – such as atherosclerosis or chronic hypertension. In physiologic states, vasodilation occurs through the serotonin mediated release of nitric oxide from endothelial cells. Additionally, it inhibits the release of norepinephrine from adrenergic nerves.[17] Serotonin is also a growth factor for some types of cells, which may give it a role in wound healing. There are various serotonin receptors.
Serotonin is metabolized mainly to 5-HIAA, chiefly by the liver. Metabolism involves first oxidation by monoamine oxidase to the corresponding aldehyde. The rate-limiting step is hydride transfer from serotonin to the flavin cofactor.[18] There follows oxidation by aldehyde dehydrogenase to 5-HIAA, the indole acetic-acid derivative. The latter is then excreted by the kidneys.
Besides mammals, serotonin is found in all bilateral animals including worms and insects,[19] as well as in fungi and in plants.[20] Serotonin’s presence in insect venoms and plant spines serves to cause pain, which is a side-effect of serotonin injection.[21][22] Serotonin is produced by pathogenic amoebae, and its effect in the human gut is diarrhea.[23] Its widespread presence in many seeds and fruits may serve to stimulate the digestive tract into expelling the seeds.[24]
Biological role[edit]
Serotonin is involved in numerous physiological processes, including sleep, thermoregulation, learning and memory, pain, (social) behavior,[25] sexual activity, feeding, motor activity, biological rhythms and possibly others.[26] In less complex animals, such as some invertebrates, serotonin regulates feeding and other processes.[27] In plants serotonin synthesis seems to be associated with stress signals.[20][28]
Cellular effects[edit]
Serotonin primarily acts through its receptors and its effects depend on which cells and tissues express these receptors (see below).[26]
Receptors[edit]
The 5-HT receptors, the receptors for serotonin, are located on the cell membrane of nerve cells and other cell types in animals, and mediate the effects of serotonin as the endogenous ligand and of a broad range of pharmaceutical and psychedelic drugs. Except for the 5-HT3 receptor, a ligand-gated ion channel, all other 5-HT receptors are G-protein-coupled receptors (also called seven-transmembrane, or heptahelical receptors) that activate an intracellular second messenger cascade.[29]
Termination[edit]
Serotonergic action is terminated primarily via uptake of 5-HT from the synapse. This is accomplished through the specific monoamine transporter for 5-HT, SERT, on the presynaptic neuron. Various agents can inhibit 5-HT reuptake, including cocaine, dextromethorphan (an antitussive), tricyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs). A 2006 study conducted by the University of Washington suggested that a newly discovered monoamine transporter, known as PMAT, may account for «a significant percentage of 5-HT clearance».[30]
Contrasting with the high-affinity SERT, the PMAT has been identified as a low-affinity transporter, with an apparent Km of 114 micromoles/l for serotonin; approximately 230 times higher than that of SERT. However, the PMAT, despite its relatively low serotonergic affinity, has a considerably higher transport ‘capacity’ than SERT, «resulting in roughly comparable uptake efficiencies to SERT in heterologous expression systems.»[30] The study also suggests some SSRIs, such as fluoxetine and sertraline anti-depressants, inhibit PMAT but at IC50 values which surpass the therapeutic plasma concentrations by up to four orders of magnitude. Therefore, SSRI monotherapy is «ineffective» in PMAT inhibition. At present, no known pharmaceuticals are known to appreciably inhibit PMAT at normal therapeutic doses. The PMAT also suggestively transports dopamine and norepinephrine, albeit at Km values even higher than that of 5-HT (330–15,000 μmoles/L).[30]
Serotonylation[edit]
Serotonin can also signal through a nonreceptor mechanism called serotonylation, in which serotonin modifies proteins.[31] This process underlies serotonin’s effects upon platelet-forming cells (thrombocytes) in which it links to the modification of signaling enzymes called GTPases that then trigger the release of vesicle contents by exocytosis.[32] A similar process underlies the pancreatic release of insulin.[31]
The effects of serotonin upon vascular smooth muscle tone – the biological function after which serotonin was originally named – depend upon the serotonylation of proteins involved in the contractile apparatus of muscle cells.[33]
| Receptor | Ki (nM)[34] | Receptor function[Note 1] |
|---|---|---|
| 5-HT1 receptor family signals via Gi/o inhibition of adenylyl cyclase. | ||
| 5-HT1A | 3.17 | Memory[vague] (agonists ↓); learning[vague] (agonists ↓); anxiety (agonists ↓); depression (agonists ↓); positive, negative, and cognitive symptoms of schizophrenia (partial agonists ↓); analgesia (agonists ↑); aggression (agonists ↓); dopamine release in the prefrontal cortex (agonists ↑); serotonin release and synthesis (agonists ↓) |
| 5-HT1B | 4.32 | Vasoconstriction (agonists ↑); aggression (agonists ↓); bone mass (↓). Serotonin autoreceptor. |
| 5-HT1D | 5.03 | Vasoconstriction (agonists ↑) |
| 5-HT1E | 7.53 | |
| 5-HT1F | 10 | |
| 5-HT2 receptor family signals via Gq activation of phospholipase C. | ||
| 5-HT2A | 11.55 | Psychedelia (agonists ↑); depression (agonists & antagonists ↓); anxiety (antagonists ↓); positive and negative symptoms of schizophrenia (antagonists ↓); norepinephrine release from the locus coeruleus (antagonists ↑); glutamate release in the prefrontal cortex (agonists ↑); dopamine in the prefrontal cortex (agonists ↑);[35] urinary bladder contractions (agonists ↑)[36] |
| 5-HT2B | 8.71 | Cardiovascular functioning (agonists increase risk of pulmonary hypertension), empathy (via von Economo neurons[37]) |
| 5-HT2C | 5.02 | Dopamine release into the mesocorticolimbic pathway (agonists ↓); acetylcholine release in the prefrontal cortex (agonists ↑); dopaminergic and noradrenergic activity in the frontal cortex (antagonists ↑);[38] appetite (agonists ↓); antipsychotic effects (agonists ↑); antidepressant effects (agonists & antagonists ↑) |
| Other 5-HT receptors | ||
| 5-HT3 | 593 | Emesis (agonists ↑); anxiolysis (antagonists ↑). |
| 5-HT4 | 125.89 | Movement of food across the GI tract (agonists ↑); memory & learning (agonists ↑); antidepressant effects (agonists ↑). Signalling via Gαs activation of adenylyl cyclase. |
| 5-HT5A | 251.2 | Memory consolidation.[39] Signals via Gi/o inhibition of adenylyl cyclase. |
| 5-HT6 | 98.41 | Cognition (antagonists ↑); antidepressant effects (agonists & antagonists ↑); anxiogenic effects (antagonists ↑[40]). Gs signalling via activating adenylyl cyclase. |
| 5-HT7 | 8.11 | Cognition (antagonists ↑); antidepressant effects (antagonists ↑). Acts by Gs signalling via activating adenylyl cyclase. |
Nervous system[edit]
The neurons of the raphe nuclei are the principal source of 5-HT release in the brain.[41] There are nine raphe nuclei, designated B1–B9, which contain the majority of serotonin-containing neurons (some scientists chose to group the nuclei raphes lineares into one nucleus), all of which are located along the midline of the brainstem, and centered on the reticular formation.[42][43] Axons from the neurons of the raphe nuclei form a neurotransmitter system reaching almost every part of the central nervous system. Axons of neurons in the lower raphe nuclei terminate in the cerebellum and spinal cord, while the axons of the higher nuclei spread out in the entire brain.
Ultrastructure and function[edit]
The serotonin nuclei may also be divided into two main groups, the rostral and caudal containing three and four nuclei respectively. The rostral group consists of the caudal linear nuclei (B8), the dorsal raphe nuclei (B6 and B7) and the median raphe nuclei (B5, B8 and B9), that project into multiple cortical and subcortical structures. The caudal group consists of the nucleus raphe magnus (B3), raphe obscurus nucleus (B2), raphe pallidus nucleus (B1), and lateral medullary reticular formation, that project into the brainstem.[44]
The serotonergic pathway is involved in sensorimotor function, with pathways projecting both into cortical (Dorsal and Median Raphe Nuclei), subcortical, and spinal areas involved in motor activity. Pharmacological manipulation suggests that serotonergic activity increases with motor activity while firing rates of serotonergic neurons increase with intense visual stimuli. Animal models suggest that kainate signaling negatively regulates serotonin actions in the retina, with possible implications for the control of the visual system.[45] The descending projections form a pathway that inhibits pain called the «descending inhibitory pathway» that may be relevant to a disorder such as fibromyalgia, migraine, and other pain disorders, and the efficacy of antidepressants in them.[46]
Serotonergic projections from the caudal nuclei are involved in regulating mood and emotion, and hypo-[47] or hyper-serotonergic[48] states may be involved in depression and sickness behavior.
Microanatomy[edit]
Serotonin is released into the synapse, or space between neurons, and diffuses over a relatively wide gap (>20 nm) to activate 5-HT receptors located on the dendrites, cell bodies, and presynaptic terminals of adjacent neurons.
When humans smell food, dopamine is released to increase the appetite. But, unlike in worms, serotonin does not increase anticipatory behaviour in humans; instead, the serotonin released while consuming activates 5-HT2C receptors on dopamine-producing cells. This halts their dopamine release, and thereby serotonin decreases appetite. Drugs that block 5-HT2C receptors make the body unable to recognize when it is no longer hungry or otherwise in need of nutrients, and are associated with weight gain,[49] especially in people with a low number of receptors.[50] The expression of 5-HT2C receptors in the hippocampus follows a diurnal rhythm,[51] just as the serotonin release in the ventromedial nucleus, which is characterised by a peak at morning when the motivation to eat is strongest.[52]
In macaques, alpha males have twice the level of serotonin in the brain as subordinate males and females (measured by the concentration of 5-HIAA in the cerebrospinal fluid (CSF)). Dominance status and CSF serotonin levels appear to be positively correlated. When dominant males were removed from such groups, subordinate males begin competing for dominance. Once new dominance hierarchies were established, serotonin levels of the new dominant individuals also increased to double those in subordinate males and females. The reason why serotonin levels are only high in dominant males, but not dominant females has not yet been established.[53]
In humans, levels of 5-HT1A receptor inhibition in the brain show negative correlation with aggression,[54] and a mutation in the gene that codes for the 5-HT2A receptor may double the risk of suicide for those with that genotype.[55] Serotonin in the brain is not usually degraded after use, but is collected by serotonergic neurons by serotonin transporters on their cell surfaces. Studies have revealed nearly 10% of total variance in anxiety-related personality depends on variations in the description of where, when and how many serotonin transporters the neurons should deploy.[56]
Psychological influences[edit]
Serotonin has been implicated in cognition, mood, anxiety and psychosis, but strong clarity has not been achieved.[57][58]
Autism spectrum disorder (ASD)[edit]
In regards to research for neurotransmitters and effects on patients with Autism Spectrum Disorder (ASD), 5-HT has been studied the most in terms of research efforts and investigations.[59] As noted, 5-HT signaling does facilitate many neural processes including that of neurogenesis, cell migration and survival, synaptogenesis, and synaptic plasticity.[59] It was noted that 45% of tested ASD subjects contained high levels of 5-HT in their blood.[59] In addition, investigations performed on ASD-like animal models reported that hyperserotonemia significantly reduced the motivation for social interest through inhibition of separation distress, which could be related in the ASD patients that have social impairments.[59]
Outside the nervous system[edit]
In the digestive tract (emetic)[edit]
Serotonin regulates gastrointestinal function. The gut is surrounded by enterochromaffin cells, which release serotonin in response to food in the lumen. This makes the gut contract around the food. Platelets in the veins draining the gut collect excess serotonin. There are often serotonin abnormalities in gastrointestinal disorders such as constipation and irritable bowel syndrome.[60]
If irritants are present in the food, the enterochromaffin cells release more serotonin to make the gut move faster, i.e., to cause diarrhea, so the gut is emptied of the noxious substance. If serotonin is released in the blood faster than the platelets can absorb it, the level of free serotonin in the blood is increased. This activates 5-HT3 receptors in the chemoreceptor trigger zone that stimulate vomiting.[61] Thus, drugs and toxins stimulate serotonin release from enterochromaffin cells in the gut wall. The enterochromaffin cells not only react to bad food but are also very sensitive to irradiation and cancer chemotherapy. Drugs that block 5HT3 are very effective in controlling the nausea and vomiting produced by cancer treatment, and are considered the gold standard for this purpose.[62]
Bone metabolism[edit]
In mice and humans, alterations in serotonin levels and signalling have been shown to regulate bone mass.[63][64][65][66] Mice that lack brain serotonin have osteopenia, while mice that lack gut serotonin have high bone density. In humans, increased blood serotonin levels have been shown to be significant negative predictor of low bone density. Serotonin can also be synthesized, albeit at very low levels, in the bone cells. It mediates its actions on bone cells using three different receptors. Through 5-HT1B receptors, it negatively regulates bone mass, while it does so positively through 5-HT2B receptors and 5-HT2C receptors. There is very delicate balance between physiological role of gut serotonin and its pathology. Increase in the extracellular content of serotonin results in a complex relay of signals in the osteoblasts culminating in FoxO1/ Creb and ATF4 dependent transcriptional events.[67] Following the 2008 findings that gut serotonin regulates bone mass, the mechanistic investigations into what regulates serotonin synthesis from the gut in the regulation of bone mass have started. Piezo1 has been shown to sense RNA in the gut and relay this information through serotonin synthesis to the bone by acting as a sensor of single-stranded RNA (ssRNA) governing 5-HT production. Intestinal epithelium-specific deletion of mouse Piezo1 profoundly disturbed gut peristalsis, impeded experimental colitis, and suppressed serum 5-HT levels. Because of systemic 5-HT deficiency, conditional knockout of Piezo1 increased bone formation. Notably, fecal ssRNA was identified as a natural Piezo1 ligand, and ssRNA-stimulated 5-HT synthesis from the gut was evoked in a MyD88/TRIF-independent manner. Colonic infusion of RNase A suppressed gut motility and increased bone mass. These findings suggest gut ssRNA as a master determinant of systemic 5-HT levels, indicating the ssRNA-Piezo1 axis as a potential prophylactic target for treatment of bone and gut disorders. Studies in 2008, 2010 and 2019 have opened the potential for serotonin research to treat bone mass disorders.[68][69]
Organ development[edit]
Since serotonin signals resource availability it is not surprising that it affects organ development. Many human and animal studies have shown that nutrition in early life can influence, in adulthood, such things as body fatness, blood lipids, blood pressure, atherosclerosis, behavior, learning, and longevity.[70][71][72] Rodent experiment shows that neonatal exposure to SSRIs makes persistent changes in the serotonergic transmission of the brain resulting in behavioral changes,[73][74] which are reversed by treatment with antidepressants.[75] By treating normal and knockout mice lacking the serotonin transporter with fluoxetine scientists showed that normal emotional reactions in adulthood, like a short latency to escape foot shocks and inclination to explore new environments were dependent on active serotonin transporters during the neonatal period.[76][77]
Human serotonin can also act as a growth factor directly. Liver damage increases cellular expression of 5-HT2A and 5-HT2B receptors, mediating liver compensatory regrowth (see Liver § Regeneration and transplantation)[78] Serotonin present in the blood then stimulates cellular growth to repair liver damage.[79]
5HT2B receptors also activate osteocytes, which build up bone[80] However, serotonin also inhibits osteoblasts, through 5-HT1B receptors.[81]
Cardiovascular growth factor[edit]
Serotonin, in addition, evokes endothelial nitric oxide synthase activation and stimulates, through a 5-HT1B receptor-mediated mechanism, the phosphorylation of p44/p42 mitogen-activated protein kinase activation in bovine aortic endothelial cell cultures.[clarification needed][82] In blood, serotonin is collected from plasma by platelets, which store it. It is thus active wherever platelets bind in damaged tissue, as a vasoconstrictor to stop bleeding, and also as a fibrocyte mitotic (growth factor), to aid healing.[83]
Skin[edit]
Serotonin is also produced by Merkel cells which are part of the somatosensory system.[84]
Lungs[edit]
Pulmonary neuroendocrine cells are specialized epithelial cells that occur as solitary cells or as clusters called neuroepithelial bodies in the lung. Pulmonary neuroendocrine cells are also known as Kulchitsky cells or K cells.[85]
Pharmacology[edit]
Several classes of drugs target the 5-HT system, including some antidepressants, antipsychotics, anxiolytics, antiemetics, and antimigraine drugs, as well as, the psychedelic drugs and empathogens.
Mechanism of action[edit]
At rest, serotonin is stored within the vesicles of presynaptic neurons. When stimulated by nerve impulses, serotonin is released as a neurotransmitter into the synapse, reversibly binding to the postsynaptic receptor to induce a nerve impulse on the postsynaptic neuron. Serotonin can also bind to auto-receptors on the presynaptic neuron to regulate the synthesis and release of serotonin. Normally serotonin is taken back into the presynaptic neuron to stop its action, then reused or broken down by monoamine oxidase.[86]
Psychedelic drugs[edit]
The serotonergic psychedelic drugs psilocin/psilocybin, DMT, mescaline, psychedelic mushroom and LSD are agonists, primarily at 5HT2A/2C receptors.[87][88][89] The empathogen-entactogen MDMA releases serotonin from synaptic vesicles of neurons.[90]
Antidepressants[edit]
Drugs that alter serotonin levels are used in treating depression, generalized anxiety disorder, and social phobia. Monoamine oxidase inhibitors (MAOIs) prevent the breakdown of monoamine neurotransmitters (including serotonin), and therefore increase concentrations of the neurotransmitter in the brain. MAOI therapy is associated with many adverse drug reactions, and patients are at risk of hypertensive emergency triggered by foods with high tyramine content, and certain drugs. Some drugs inhibit the re-uptake of serotonin, making it stay in the synaptic cleft longer. The tricyclic antidepressants (TCAs) inhibit the reuptake of both serotonin and norepinephrine. The newer selective serotonin reuptake inhibitors (SSRIs) have fewer side-effects and fewer interactions with other drugs.[91]
Certain SSRI medications have been shown to lower serotonin levels below the baseline after chronic use, despite initial increases.[92] The 5-HTTLPR gene codes for the number of serotonin transporters in the brain, with more serotonin transporters causing decreased duration and magnitude of serotonergic signaling.[93] The 5-HTTLPR polymorphism (l/l) causing more serotonin transporters to be formed is also found to be more resilient against depression and anxiety.[94][95]
Serotonin syndrome[edit]
Extremely high levels of serotonin can cause a condition known as serotonin syndrome, with toxic and potentially fatal effects. In practice, such toxic levels are essentially impossible to reach through an overdose of a single antidepressant drug, but require a combination of serotonergic agents, such as an SSRI with a MAOI, which may occur in therapeutic doses.[96][97] The intensity of the symptoms of serotonin syndrome vary over a wide spectrum, and the milder forms are seen even at nontoxic levels.[98] It is estimated that 14% of patients experiencing serotonin syndrome overdose on SSRIs; meanwhile the fatality rate is between 2% to 12%.[96][99][100]
Antiemetics[edit]
Some 5-HT3 antagonists, such as ondansetron, granisetron, and tropisetron, are important antiemetic agents. They are particularly important in treating the nausea and vomiting that occur during anticancer chemotherapy using cytotoxic drugs. Another application is in the treatment of postoperative nausea and vomiting.
Other[edit]
Some serotonergic agonist drugs cause fibrosis anywhere in the body, particularly the syndrome of retroperitoneal fibrosis, as well as cardiac valve fibrosis.[101]
In the past, three groups of serotonergic drugs have been epidemiologically linked with these syndromes. These are the serotonergic vasoconstrictive antimigraine drugs (ergotamine and methysergide),[101] the serotonergic appetite suppressant drugs (fenfluramine, chlorphentermine, and aminorex), and certain anti-Parkinsonian dopaminergic agonists, which also stimulate serotonergic 5-HT2B receptors. These include pergolide and cabergoline, but not the more dopamine-specific lisuride.[102]
As with fenfluramine, some of these drugs have been withdrawn from the market after groups taking them showed a statistical increase of one or more of the side effects described. An example is pergolide. The drug was declining in use since it was reported in 2003 to be associated with cardiac fibrosis.[103]
Two independent studies published in The New England Journal of Medicine in January 2007 implicated pergolide, along with cabergoline, in causing valvular heart disease.[104][105] As a result of this, the FDA removed pergolide from the United States market in March 2007.[106] (Since cabergoline is not approved in the United States for Parkinson’s Disease, but for hyperprolactinemia, the drug remains on the market. Treatment for hyperprolactinemia requires lower doses than that for Parkinson’s Disease, diminishing the risk of valvular heart disease).[107]
Methyl-tryptamines and hallucinogens[edit]
For details on tryptamine neurotransmitters in humans, see Trace amine.
Several plants contain serotonin together with a family of related tryptamines that are methylated at the amino (NH2) and (OH) groups, are N-oxides, or miss the OH group. These compounds do reach the brain, although some portion of them are metabolized by monoamine oxidase enzymes (mainly MAO-A) in the liver. Examples are plants from the genus Anadenanthera that are used in the hallucinogenic yopo snuff. These compounds are widely present in the leaves of many plants, and may serve as deterrents for animal ingestion. Serotonin occurs in several mushrooms of the genus Panaeolus.[108]
Comparative biology and evolution[edit]
Unicellular organisms[edit]
Serotonin is used by a variety of single-cell organisms for various purposes. SSRIs have been found to be toxic to algae.[109] The gastrointestinal parasite Entamoeba histolytica secretes serotonin, causing a sustained secretory diarrhea in some people.[23][110] Patients infected with E. histolytica have been found to have highly elevated serum serotonin levels, which returned to normal following resolution of the infection.[111] E. histolytica also responds to the presence of serotonin by becoming more virulent.[112] This means serotonin secretion not only serves to increase the spread of enteamoebas by giving the host diarrhea but also serves to coordinate their behaviour according to their population density, a phenomenon known as quorum sensing. Outside the gut of a host, there is nothing that the entoamoebas provoke to release serotonin, hence the serotonin concentration is very low. Low serotonin signals to the entoamoebas they are outside a host and they become less virulent to conserve energy. When they enter a new host, they multiply in the gut, and become more virulent as the enterochromaffine cells get provoked by them and the serotonin concentration increases.
Edible plants and mushrooms[edit]
In drying seeds, serotonin production is a way to get rid of the buildup of poisonous ammonia. The ammonia is collected and placed in the indole part of L-tryptophan, which is then decarboxylated by tryptophan decarboxylase to give tryptamine, which is then hydroxylated by a cytochrome P450 monooxygenase, yielding serotonin.[113]
However, since serotonin is a major gastrointestinal tract modulator, it may be produced in the fruits of plants as a way of speeding the passage of seeds through the digestive tract, in the same way as many well-known seed and fruit associated laxatives. Serotonin is found in mushrooms, fruits, and vegetables. The highest values of 25–400 mg/kg have been found in nuts of the walnut (Juglans) and hickory (Carya) genera. Serotonin concentrations of 3–30 mg/kg have been found in plantains, pineapples, banana, kiwifruit, plums, and tomatoes. Moderate levels from 0.1–3 mg/kg have been found in a wide range of tested vegetables.[24][20]
Serotonin is one compound of the poison contained in stinging nettles (Urtica dioica), where it causes pain on injection in the same manner as its presence in insect venoms (see below).[22] It is also naturally found in Paramuricea clavata, or the Red Sea Fan.[114]
Serotonin and tryptophan have been found in chocolate with varying cocoa contents. The highest serotonin content (2.93 µg/g) was found in chocolate with 85% cocoa, and the highest tryptophan content (13.27–13.34 µg/g) was found in 70–85% cocoa. The intermediate in the synthesis from tryptophan to serotonin, 5-hydroxytryptophan, was not found.[115]
Root development in Arabidopsis thaliana is stimulated and modulated by serotonin — in various ways at various concentrations.[116]
Serotonin serves as a plant defense chemical against fungi. When infected with Fusarium crown rot (Fusarium pseudograminearum), wheat (Triticum aestivum) greatly increases its production of tryptophan to synthesize new serotonin.[117] The function of this is poorly understood[117] but wheat also produces serotonin when infected by Stagonospora nodorum — in that case to retard spore production.[118] The model cereal Brachypodium distachyon — used as a research substitute for wheat and other production cereals — also produces serotonin, coumaroyl-serotonin, and feruloyl-serotonin in response to F. graminearum. This produces a slight antimicrobial effect. B. distachyon produces more serotonin (and conjugates) in response to deoxynivalenol (DON)-producing F. graminearum than non-DON-producing.[119] Solanum lycopersicum produces many AA conjugates — including several of serotonin — in its leaves, stems, and roots in response to Ralstonia solanacearum infection.[120]
Invertebrates[edit]
Serotonin functions as a neurotransmitter in the nervous systems of most animals.
Nematodes[edit]
For example, in the roundworm Caenorhabditis elegans, which feeds on bacteria, serotonin is released as a signal in response to positive events, such as finding a new source of food or in male animals finding a female with which to mate.[121] When a well-fed worm feels bacteria on its cuticle, dopamine is released, which slows it down; if it is starved, serotonin also is released, which slows the animal down further. This mechanism increases the amount of time animals spend in the presence of food.[122] The released serotonin activates the muscles used for feeding, while octopamine suppresses them.[123][124] Serotonin diffuses to serotonin-sensitive neurons, which control the animal’s perception of nutrient availability.
Decapods[edit]
If lobsters are injected with serotonin, they behave like dominant individuals whereas octopamine causes subordinate behavior.[25] A crayfish that is frightened may flip its tail to flee, and the effect of serotonin on this behavior depends largely on the animal’s social status. Serotonin inhibits the fleeing reaction in subordinates, but enhances it in socially dominant or isolated individuals. The reason for this is social experience alters the proportion between serotonin receptors (5-HT receptors) that have opposing effects on the fight-or-flight response.[clarification needed] The effect of 5-HT1 receptors predominates in subordinate animals, while 5-HT2 receptors predominates in dominants.[125]
In venoms[edit]
Serotonin is a common component of invertebrate venoms, salivary glands, nervous tissues, and various other tissues, across molluscs, insects, crustaceans, scorpions, various kinds of worms, and jellyfish.[22] Adult Rhodnius prolixus — hematophagous on vertebrates — secrete lipocalins into the wound during feeding. In 2003 these lipocalins were demonstrated to sequester serotonin to prevent vasoconstriction (and possibly coagulation) in the host.[126]
Insects[edit]
Serotonin is evolutionarily conserved and appears across the animal kingdom. It is seen in insect processes in roles similar to in the human central nervous system, such as memory, appetite, sleep, and behavior.[127][19] Some circuits in mushroom bodies are serotonergic.[128] (See specific Drosophila example below, §Dipterans.)
Acrididae[edit]
Locust swarming is initiated but not maintained by serotonin,[129] with release being triggered by tactile contact between individuals.[130] This transforms social preference from aversion to a gregarious state that enables coherent groups.[131][130][129] Learning in flies and honeybees is affected by the presence of serotonin.[132][133]
Role in insecticides[edit]
Insect 5-HT receptors have similar sequences to the vertebrate versions, but pharmacological differences have been seen. Invertebrate drug response has been far less characterized than mammalian pharmacology and the potential for species selective insecticides has been discussed.[134]
Hymenopterans[edit]
Wasps and hornets have serotonin in their venom,[135] which causes pain and inflammation[21][22] as do scorpions.[136][22] Pheidole dentata takes on more and more tasks in the colony as it gets older, which requires it to respond to more and more olfactory cues in the course of performing them. This olfactory response broadening was demonstrated to go along with increased serotonin and dopamine, but not octopamine in 2006.[137]
Dipterans[edit]
If flies are fed serotonin, they are more aggressive; flies depleted of serotonin still exhibit aggression, but they do so much less frequently.[138] In their crops it plays a vital role in digestive motility produced by contraction. Serotonin that acts on the crop is exogenous to the crop itself and 2012 research suggested that it probably originated in the serotonin neural plexus in the thoracic-abdominal synganglion.[139] In 2011 a Drosophila serotonergic mushroom body was found to work in concert with Amnesiac to form memories.[128] In 2007 serotonin was found to promote aggression in Diptera, which was counteracted by neuropeptide F — a surprising find given that they both promote courtship, which is usually similar to aggression in most respects.[128]
Vertebrates[edit]
Serotonin, also referred to as 5-hydroxytryptamine (5-HT), is a neurotransmitter most known for its involvement in mood disorders in humans. It is also a widely present neuromodulator among vertebrates and invertebrates.[140] Serotonin has been found having associations with many physiological systems such as cardiovascular, thermoregulation, and behavioral functions, including: circadian rhythm, appetite, aggressive and sexual behavior, sensorimotor reactivity and learning, and pain sensitivity.[141] Serotonin’s function in neurological systems along with specific behaviors among vertebrates found to be strongly associated with serotonin will be further discussed. Two relevant case studies are also mentioned regarding serotonin development involving teleost fish and mice.
In mammals, 5-HT is highly concentrated in the substantia nigra, ventral tegmental area and raphe nuclei. Lesser concentrated areas include other brain regions and the spinal cord.[140] 5-HT neurons are also shown to be highly branched, indicating that they are structurally prominent for influencing multiple areas of the CNS at the same time, although this trend is exclusive solely to mammals.[141]
5-HT System in Vertebrates[edit]
Vertebrates are multicellular organisms in the phylum Chordata that possess a backbone and a nervous system. This includes mammals, fish, reptiles, birds, etc. In humans, the nervous system is composed of the central and peripheral nervous system, with little known about the specific mechanisms of neurotransmitters in most other vertebrates. However, it is known that while serotonin is involved in stress and behavioral responses, it is also important in cognitive functions.[140] Brain organization in most vertebrates includes 5-HT cells in the hindbrain.[140] In addition to this, 5-HT is often found in other sections of the brain in non-placental vertebrates, including the basal forebrain and pretectum.[142] Since location of serotonin receptors contribute to behavioral responses, this suggests serotonin is part of specific pathways in non-placental vertebrates that are not present in amniotic organisms.[143] Teleost fish and mice are organisms most often used to study the connection between serotonin and vertebrate behavior. Both organisms show similarities in the effect of serotonin on behavior, but differ in the mechanism in which the responses occur.
Dogs / Canine species[edit]
There are few studies of serotonin in dogs. One study reported serotonin values were higher at dawn than at dusk.[144] In another study, serum 5-HT levels did not seem to be associated with dogs’ behavioural response to a stressful situation.[145] Urinary serotonin/creatinine ratio in bitches tended to be higher 4 weeks after surgery. In addition, serotonin was positively correlated with both cortisol and progesterone but not with testosterone after ovariohysterectomy.[146]
Teleost Fish[edit]
Like non-placental vertebrates, teleost fish also possess 5-HT cells in other sections of the brain, including the basal forebrain.[142] Danio rerio (zebra fish) are a species of teleost fish often used for studying serotonin within the brain. Despite much being unknown about serotonergic systems in vertebrates, the importance in moderating stress and social interaction is known.[147] It is hypothesized that AVT and CRF cooperate with serotonin in the hypothalamic-pituitary-interrenal axis.[142] These neuropeptides influence the plasticity of the teleost, affecting its ability to change and respond to its environment. Subordinate fish in social settings show a drastic increase in 5-HT concentrations.[147] High levels of 5-HT long term influence the inhibition of aggression in subordinate fish.[147]
Mice[edit]
Researchers at the Department of Pharmacology and Medical Chemistry used serotonergic drugs on male mice to study the effects of selected drugs on their behavior.[148] Mice in isolation exhibit increased levels of agonistic behavior towards one another. Results found that serotonergic drugs reduce aggression in isolated mice while simultaneously increasing social interaction.[148] Each of the treatments use a different mechanism for targeting aggression, but ultimately all have the same outcome. While the study shows that serotonergic drugs successfully target serotonin receptors, it does not show specifics of the mechanisms that affect behavior, as all types of drugs tended to reduce aggression in isolated male mice.[148] Aggressive mice kept out of isolation may respond differently to changes in serotonin reuptake.
Behavior[edit]
Like in humans, serotonin is extremely involved in regulating behavior in most other vertebrates. This includes not only response and social behaviors, but also influencing mood. Defects in serotonin pathways can lead to intense variations in mood, as well as symptoms of mood disorders, which can be present in more than just humans.
[edit]
One of the most researched aspects of social interaction in which serotonin is involved is aggression. Aggression is regulated by the 5-HT system, as serotonin levels can both induce or inhibit aggressive behaviors, as seen in mice (see section on Mice) and crabs.[148] While this is widely accepted, it is unknown if serotonin interacts directly or indirectly with parts of the brain influencing aggression and other behaviors.[140] Studies of serotonin levels show that they drastically increase and decrease during social interactions, and they generally correlate with inhibiting or inciting aggressive behavior.[149] The exact mechanism of serotonin influencing social behaviors is unknown, as pathways in the 5-HT system in various vertebrates can differ greatly.[140]
Response to Stimuli[edit]
Serotonin is important in environmental response pathways, along with other neurotransmitters.[150] Specifically, it has been found to be involved in auditory processing in social settings, as primary sensory systems are connected to social interactions.[151] Serotonin is found in the IC structure of the midbrain, which processes specie specific and non-specific social interactions and vocalizations.[151] It also receives acoustic projections that convey signals to auditory processing regions.[151] Research has proposed that serotonin shapes the auditory information being received by the IC and therefore is influential in the responses to auditory stimuli.[151] This can influence how an organism responds to the sounds of predatory or other impactful species in their environment, as serotonin uptake can influence aggression and/or social interaction.
Mood[edit]
We can describe mood not as specific to an emotional status, but as associated with a relatively long-lasting emotional state. Serotonin’s association with mood is most known for various forms of depression and bipolar disorders in humans.[141] Disorders caused by serotonergic activity potentially contribute to the many symptoms of major depression, such as overall mood, activity, suicidal thoughts and sexual and cognitive dysfunction. Selective serotonin reuptake inhibitors (SSRI’s) are a class of drugs demonstrated to be an effective treatment in major depressive disorder and are the most prescribed class of antidepressants. SSRI’s function is to block the reuptake of serotonin, making more serotonin available to absorb by the receiving neuron. Animals have been studied for decades in order to understand depressive behavior among species. One of the most familiar studies, the forced swimming test (FST), was performed to measure potential antidepressant activity.[141] Rats were placed in an inescapable container of water, at which point time spent immobile and number of active behaviors (such as splashing or climbing) were compared before and after a panel of anti-depressant drugs were administered. Antidepressants that selectively inhibit NE reuptake were shown to reduce immobility and selectively increase climbing without affecting swimming. However, results of the SSRI’s also show reduced immobility but increased swimming without affecting climbing. This study demonstrated the importance of behavioral tests for antidepressants, as they can detect drugs with an effect on core behavior along with behavioral components of species.[141]
Growth and reproduction[edit]
In the nematode C. elegans, artificial depletion of serotonin or the increase of octopamine cues behavior typical of a low-food environment: C. elegans becomes more active, and mating and egg-laying are suppressed, while the opposite occurs if serotonin is increased or octopamine is decreased in this animal.[27] Serotonin is necessary for normal nematode male mating behavior,[152] and the inclination to leave food to search for a mate.[153] The serotonergic signaling used to adapt the worm’s behaviour to fast changes in the environment affects insulin-like signaling and the TGF beta signaling pathway,[154] which control long-term adaption.
In the fruit fly insulin both regulates blood sugar as well as acting as a growth factor. Thus, in the fruit fly, serotonergic neurons regulate the adult body size by affecting insulin secretion.[155][156] Serotonin has also been identified as the trigger for swarm behavior in locusts.[131] In humans, though insulin regulates blood sugar and IGF regulates growth, serotonin controls the release of both hormones, modulating insulin release from the beta cells in the pancreas through serotonylation of GTPase signaling proteins.[31] Exposure to SSRIs during pregnancy reduces fetal growth.[157]
Genetically altered C. elegans worms that lack serotonin have an increased reproductive lifespan, may become obese, and sometimes present with arrested development at a dormant larval state.[158][159]
Aging and age-related phenotypes[edit]
Serotonin is known to regulate aging, learning and memory. The first evidence comes from the study of longevity in C. elegans.[154] During early phase of aging[vague], the level of serotonin increases, which alters locomotory behaviors and associative memory.[160] The effect is restored by mutations and drugs (including mianserin and methiothepin) that inhibit serotonin receptors. The observation does not contradict with the notion that the serotonin level goes down in mammals and humans, which is typically seen in late but not early[vague] phase of aging.
Biochemical mechanisms[edit]
Biosynthesis[edit]
The pathway for the synthesis of serotonin from tryptophan.
In animals including humans, serotonin is synthesized from the amino acid L-tryptophan by a short metabolic pathway consisting of two enzymes, tryptophan hydroxylase (TPH) and aromatic amino acid decarboxylase (DDC), and the coenzyme pyridoxal phosphate. The TPH-mediated reaction is the rate-limiting step in the pathway.
TPH has been shown to exist in two forms: TPH1, found in several tissues, and TPH2, which is a neuron-specific isoform.[161]
Serotonin can be synthesized from tryptophan in the lab using Aspergillus niger and Psilocybe coprophila as catalysts. The first phase to 5-hydroxytryptophan would require letting tryptophan sit in ethanol and water for 7 days, then mixing in enough HCl (or other acid) to bring the pH to 3, and then adding NaOH to make a pH of 13 for 1 hour. Asperigillus niger would be the catalyst for this first phase. The second phase to synthesizing tryptophan itself from the 5-hydroxytryptophan intermediate would require adding ethanol and water, and letting sit for 30 days this time. The next two steps would be the same as the first phase: adding HCl to make the pH = 3, and then adding NaOH to make the pH very basic at 13 for 1 hour. This phase uses the Psilocybe coprophila as the catalyst for the reaction.[162]
Serotonin taken orally does not pass into the serotonergic pathways of the central nervous system, because it does not cross the blood–brain barrier.[9] However, tryptophan and its metabolite 5-hydroxytryptophan (5-HTP), from which serotonin is synthesized, do cross the blood–brain barrier. These agents are available as dietary supplements and in various foods, and may be effective serotonergic agents.
One product of serotonin breakdown is 5-hydroxyindoleacetic acid (5-HIAA), which is excreted in the urine. Serotonin and 5-HIAA are sometimes produced in excess amounts by certain tumors or cancers, and levels of these substances may be measured in the urine to test for these tumors.
Analytical chemistry[edit]
Indium tin oxide is recommended for the electrode material in electrochemical investigation of concentrations produced, detected, or consumed by microbes.[163] A mass spectrometry technique was developed in 1994 to measure the molecular weight of both natural and synthetic serotonins.[164]
History and etymology[edit]
It had been known to physiologists for over a century that a vasoconstrictor material appears in serum when blood was allowed to clot.[165] In 1935, Italian Vittorio Erspamer showed an extract from enterochromaffin cells made intestines contract. Some believed it contained adrenaline, but two years later, Erspamer was able to show it was a previously unknown amine, which he named «enteramine».[166][167] In 1948, Maurice M. Rapport, Arda Green, and Irvine Page of the Cleveland Clinic discovered a vasoconstrictor substance in blood serum, and since it was a serum agent affecting vascular tone, they named it serotonin.[168]
In 1952, enteramine was shown to be the same substance as serotonin, and as the broad range of physiological roles was elucidated, the abbreviation 5-HT of the proper chemical name 5-hydroxytryptamine became the preferred name in the pharmacological field.[169] Synonyms of serotonin include: 5-hydroxytriptamine, thrombotin, enteramin, substance DS, and 3-(β-Aminoethyl)-5-hydroxyindole.[170] In 1953, Betty Twarog and Page discovered serotonin in the central nervous system.[171] Page regarded Erspamer’s work on Octopus vulgaris, Discoglossus pictus, Hexaplex trunculus, Bolinus brandaris, Sepia, Mytilus, and Ostrea as valid and fundamental to understanding this newly identified substance, but regarded his earlier results in various models — especially those from rat blood — to be too confounded by the presence of other MAs, including some other vasoactives.[172]
See also[edit]
- HIOC
Notes[edit]
- ^ References for the functions of these receptors are available on the wikipedia pages for the specific receptor in question
References[edit]
- ^ Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (©1994–2011 ACD/Labs)
- ^ Mazák K, Dóczy V, Kökösi J, Noszál B (April 2009). «Proton speciation and microspeciation of serotonin and 5-hydroxytryptophan». Chemistry & Biodiversity. 6 (4): 578–590. doi:10.1002/cbdv.200800087. PMID 19353542. S2CID 20543931.
- ^ Pietra S (1958). «[Indolic derivatives. II. A new way to synthesize serotonin]». Il Farmaco; Edizione Scientifica (in Italian). 13 (1): 75–79. PMID 13524273.
- ^ Erspamer V (1952). «Ricerche preliminari sulle indolalchilamine e sulle fenilalchilamine degli estratti di pelle di Anfibio». Ricerca Scientifica. 22: 694–702.
- ^ Tammisto T (1967). «Increased toxicity of 5-hydroxytryptamine by ethanol in rats and mice». Annales Medicinae Experimentalis et Biologiae Fenniae. 46 (3): 382–384. PMID 5734241.
- ^ Jones D (2003) [1917]. Roach P, Hartmann J, Setter J (eds.). English Pronouncing Dictionary. Cambridge: Cambridge University Press. ISBN 978-3-12-539683-8.
- ^ «Serotonin». Dictionary.com Unabridged (Online). n.d.
- ^ «Serotonin». Merriam-Webster Dictionary.
- ^ a b Young SN (November 2007). «How to increase serotonin in the human brain without drugs». Journal of Psychiatry & Neuroscience. 32 (6): 394–399. PMC 2077351. PMID 18043762.
- ^ «Microbes Help Produce Serotonin in Gut». California Institute of Technology. 9 April 2015. Retrieved 3 June 2022.
- ^ González-Flores D, Velardo B, Garrido M, et al. (2011). «Ingestion of Japanese plums (Prunus salicina Lindl. cv. Crimson Globe) increases the urinary 6-sulfatoxymelatonin and total antioxidant capacity levels in young, middle-aged and elderly humans: Nutritional and functional characterization of their content». Journal of Food and Nutrition Research. 50 (4): 229–236.
- ^ Schlienger RG, Meier CR (2003). «Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction?». American Journal of Cardiovascular Drugs: Drugs, Devices, and Other Interventions. 3 (3): 149–162. doi:10.2165/00129784-200303030-00001. PMID 14727927. S2CID 23986530.
- ^ King MW. «Serotonin». The Medical Biochemistry Page. Indiana University School of Medicine. Retrieved 1 December 2009.
- ^ Berger M, Gray JA, Roth BL (2009). «The expanded biology of serotonin». Annual Review of Medicine. 60: 355–366. doi:10.1146/annurev.med.60.042307.110802. PMC 5864293. PMID 19630576.
- ^ Kling A (2013). 5-HT2A: a serotonin receptor with a possible role in joint diseases (PDF) (Thesis). Umeå Universitet. ISBN 978-91-7459-549-9.
- ^ Yano JM, Yu K, Donaldson GP, et al. (April 2015). «Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis». Cell. 161 (2): 264–276. doi:10.1016/j.cell.2015.02.047. PMC 4393509. PMID 25860609.
- ^ Vanhoutte PM (February 1987). «Serotonin and the vascular wall». International Journal of Cardiology. 14 (2): 189–203. doi:10.1016/0167-5273(87)90008-8. PMID 3818135.
- ^ Prah A, Purg M, Stare J, et al. (September 2020). «How Monoamine Oxidase A Decomposes Serotonin: An Empirical Valence Bond Simulation of the Reactive Step». The Journal of Physical Chemistry B. 124 (38): 8259–8265. doi:10.1021/acs.jpcb.0c06502. PMC 7520887. PMID 32845149.
- ^ a b Huser A, Rohwedder A, Apostolopoulou AA, Widmann A, Pfitzenmaier JE, Maiolo EM, et al. (2012). Zars T (ed.). «The serotonergic central nervous system of the Drosophila larva: anatomy and behavioral function». PLOS ONE. 7 (10): e47518. Bibcode:2012PLoSO…747518H. doi:10.1371/journal.pone.0047518. PMC 3474743. PMID 23082175.
- ^ a b c Ramakrishna A, Giridhar P, Ravishankar GA (June 2011). «Phytoserotonin: a review». Plant Signaling & Behavior. 6 (6): 800–809. doi:10.4161/psb.6.6.15242. PMC 3218476. PMID 21617371.
- ^ a b Chen J, Lariviere WR (October 2010). «The nociceptive and anti-nociceptive effects of bee venom injection and therapy: a double-edged sword». Progress in Neurobiology. 92 (2): 151–183. doi:10.1016/j.pneurobio.2010.06.006. PMC 2946189. PMID 20558236.
- ^ a b c d e Erspamer V (1966). «Occurrence of indolealkylamines in nature». 5-Hydroxytryptamine and Related Indolealkylamines. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 132–181. doi:10.1007/978-3-642-85467-5_4. ISBN 978-3-642-85469-9.
- ^ a b McGowan K, Kane A, Asarkof N, et al. (August 1983). «Entamoeba histolytica causes intestinal secretion: role of serotonin». Science. 221 (4612): 762–764. Bibcode:1983Sci…221..762M. doi:10.1126/science.6308760. PMID 6308760.
- ^ a b Feldman JM, Lee EM (October 1985). «Serotonin content of foods: effect on urinary excretion of 5-hydroxyindoleacetic acid». The American Journal of Clinical Nutrition. 42 (4): 639–643. doi:10.1093/ajcn/42.4.639. PMID 2413754.
- ^ a b Kravitz EA (September 1988). «Hormonal control of behavior: amines and the biasing of behavioral output in lobsters». Science. 241 (4874): 1775–1781. Bibcode:1988Sci…241.1775K. doi:10.1126/science.2902685. PMID 2902685.
- ^ a b Zifa E, Fillion G (September 1992). «5-Hydroxytryptamine receptors». Pharmacological Reviews. 44 (3): 401–458. PMID 1359584.
- ^ a b Srinivasan S, Sadegh L, Elle IC, et al. (June 2008). «Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms». Cell Metabolism. 7 (6): 533–544. doi:10.1016/j.cmet.2008.04.012. PMC 2495008. PMID 18522834.
- ^ Ramakrishna A, Ravishankar GA (November 2011). «Influence of abiotic stress signals on secondary metabolites in plants». Plant Signaling & Behavior. Informa. 6 (11): 1720–1731. doi:10.4161/psb.6.11.17613. PMC 3329344. PMID 22041989.
- ^ Hannon J, Hoyer D (December 2008). «Molecular biology of 5-HT receptors». Behavioural Brain Research. 195 (1): 198–213. doi:10.1016/j.bbr.2008.03.020. PMID 18571247. S2CID 46043982.
- ^ a b c Zhou M, Engel K, Wang J (January 2007). «Evidence for significant contribution of a newly identified monoamine transporter (PMAT) to serotonin uptake in the human brain». Biochemical Pharmacology. 73 (1): 147–154. doi:10.1016/j.bcp.2006.09.008. PMC 1828907. PMID 17046718.
- ^ a b c Paulmann N, Grohmann M, Voigt JP, et al. (October 2009). O’Rahilly S (ed.). «Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation». PLOS Biology. 7 (10): e1000229. doi:10.1371/journal.pbio.1000229. PMC 2760755. PMID 19859528.
- ^ Walther DJ, Peter JU, Winter S, et al. (December 2003). «Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release». Cell. 115 (7): 851–862. doi:10.1016/S0092-8674(03)01014-6. PMID 14697203. S2CID 16847296.
- ^ Watts SW, Priestley JR, Thompson JM (May 2009). «Serotonylation of vascular proteins important to contraction». PLOS ONE. 4 (5): e5682. Bibcode:2009PLoSO…4.5682W. doi:10.1371/journal.pone.0005682. PMC 2682564. PMID 19479059.
- ^ Roth BL, Driscol J (12 January 2011). «PDSP Ki Database». Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 8 November 2013. Retrieved 17 December 2013.
- ^ Bortolozzi A, Díaz-Mataix L, Scorza MC, et al. (December 2005). «The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity». Journal of Neurochemistry. 95 (6): 1597–1607. doi:10.1111/j.1471-4159.2005.03485.x. hdl:10261/33026. PMID 16277612. S2CID 18350703.
- ^ Moro C, Edwards L, Chess-Williams R (November 2016). «2Areceptor enhancement of contractile activity of the porcine urothelium and lamina propria». International Journal of Urology. 23 (11): 946–951. doi:10.1111/iju.13172. PMID 27531585.
- ^ «Von Economo neuron – NeuronBank». neuronbank.org.[unreliable medical source?]
- ^ Millan MJ, Gobert A, Lejeune F, et al. (September 2003). «The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways». The Journal of Pharmacology and Experimental Therapeutics. 306 (3): 954–964. doi:10.1124/jpet.103.051797. PMID 12750432. S2CID 18753440.
- ^ Gonzalez R, Chávez-Pascacio K, Meneses A (September 2013). «Role of 5-HT5A receptors in the consolidation of memory». Behavioural Brain Research. 252: 246–251. doi:10.1016/j.bbr.2013.05.051. PMID 23735322. S2CID 140204585.
- ^ Nautiyal KM, Hen R (2017). «Serotonin receptors in depression: from A to B». F1000Research. 6: 123. doi:10.12688/f1000research.9736.1. PMC 5302148. PMID 28232871.
- ^ Frazer A, Hensler JG (1999). «Understanding the neuroanatomical organization of serotonergic cells in the brain provides insight into the functions of this neurotransmitter». In Siegel GJ, Agranoff, Bernard W, Fisher SK, Albers RW, Uhler MD (eds.). Basic Neurochemistry (Sixth ed.). Lippincott Williams & Wilkins. ISBN 978-0-397-51820-3.
In 1964, Dahlstrom and Fuxe (discussed in [2]), using the Falck-Hillarp technique of histofluorescence, observed that the majority of serotonergic soma are found in cell body groups, which previously had been designated as the Raphe nuclei.
- ^ Binder MD, Hirokawa N (2009). encyclopedia of neuroscience. Berlin: Springer. p. 705. ISBN 978-3-540-23735-8.
- ^ The raphe nuclei group of neurons are located along the brain stem from the labels ‘Mid Brain’ to ‘Oblongata’, centered on the pons. (See relevant image.)
- ^ Müller CP, Jacobs BL, eds. (2009). Handbook of the behavioral neurobiology of serotonin (1st ed.). London: Academic. pp. 51–59. ISBN 978-0123746344.
- ^ Passos AD, Herculano AM, Oliveira KR, de Lima SM, Rocha FA, Freitas HR, et al. (October 2019). «Regulation of the Serotonergic System by Kainate in the Avian Retina». Cellular and Molecular Neurobiology. 39 (7): 1039–1049. doi:10.1007/s10571-019-00701-8. PMID 31197744. S2CID 189763144.
- ^ Sommer C (2009). «Serotonin in Pain and Pain Control». In Müller CP, Jacobs BL (eds.). Handbook of the behavioral neurobiology of serotonin (1st ed.). London: Academic. pp. 457–460. ISBN 978-0123746344.
- ^ Hensler JG (2009). «Serotonin in Mode and Emotions». In Müller CP, Jacobs BL (eds.). Handbook of the behavioral neurobiology of serotonin (1st ed.). London: Academic. pp. 367–399. ISBN 978-0123746344.
- ^ Andrews PW, Bharwani A, Lee KR, et al. (April 2015). «Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response». Neuroscience and Biobehavioral Reviews. 51: 164–188. doi:10.1016/j.neubiorev.2015.01.018. PMID 25625874. S2CID 23980182.
- ^ Stahl SM, Mignon L, Meyer JM (March 2009). «Which comes first: atypical antipsychotic treatment or cardiometabolic risk?». Acta Psychiatrica Scandinavica. 119 (3): 171–179. doi:10.1111/j.1600-0447.2008.01334.x. PMID 19178394. S2CID 24035040.
- ^ Buckland PR, Hoogendoorn B, Guy CA, et al. (March 2005). «Low gene expression conferred by association of an allele of the 5-HT2C receptor gene with antipsychotic-induced weight gain». The American Journal of Psychiatry. 162 (3): 613–615. doi:10.1176/appi.ajp.162.3.613. PMID 15741483.
- ^ Holmes MC, French KL, Seckl JR (June 1997). «Dysregulation of diurnal rhythms of serotonin 5-HT2C and corticosteroid receptor gene expression in the hippocampus with food restriction and glucocorticoids». The Journal of Neuroscience. 17 (11): 4056–4065. doi:10.1523/JNEUROSCI.17-11-04056.1997. PMC 6573558. PMID 9151722.
- ^ Leibowitz SF (1990). «The role of serotonin in eating disorders». Drugs. 39 (Suppl 3): 33–48. doi:10.2165/00003495-199000393-00005. PMID 2197074. S2CID 8612545.
- ^ McGuire, Michael (2013) «Believing, the neuroscience of fantasies, fears, and confictions» (Prometius Books)
- ^ Caspi N, Modai I, Barak P, et al. (March 2001). «Pindolol augmentation in aggressive schizophrenic patients: a double-blind crossover randomized study». International Clinical Psychopharmacology. 16 (2): 111–115. doi:10.1097/00004850-200103000-00006. PMID 11236069. S2CID 24822810.
- ^ Ito Z, Aizawa I, Takeuchi M, Tabe M, Nakamura T (December 1975). «[Proceedings: Study of gastrointestinal motility using an extraluminal force transducer. 6. Observation of gastric and duodenal motility using synthetic motilin]». Nihon Heikatsukin Gakkai Zasshi. 11 (4): 244–246. PMID 1232434.
- ^ Lesch KP, Bengel D, Heils A, et al. (November 1996). «Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region». Science. 274 (5292): 1527–1531. Bibcode:1996Sci…274.1527L. doi:10.1126/science.274.5292.1527. PMID 8929413. S2CID 35503987.
- ^ Chilmonczyk Z, Bojarski AJ, Pilc A, Sylte I (August 2015). «Functional Selectivity and Antidepressant Activity of Serotonin 1A Receptor Ligands». International Journal of Molecular Sciences. 16 (8): 18474–18506. doi:10.3390/ijms160818474. PMC 4581256. PMID 26262615.
- ^ Blier P, El Mansari M (2013). «Serotonin and beyond: therapeutics for major depression». Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 368 (1615): 20120536. doi:10.1098/rstb.2012.0536. PMC 3638389. PMID 23440470.
- ^ a b c d Eissa N, Al-Houqani M, Sadeq A, Ojha SK, Sasse A, Sadek B (16 May 2018). «Current Enlightenment About Etiology and Pharmacological Treatment of Autism Spectrum Disorder». Frontiers in Neuroscience. 12: 304. doi:10.3389/fnins.2018.00304. PMC 5964170. PMID 29867317.
- ^ Beattie DT, Smith JA (May 2008). «Serotonin pharmacology in the gastrointestinal tract: a review». Naunyn-Schmiedeberg’s Archives of Pharmacology. 377 (3): 181–203. doi:10.1007/s00210-008-0276-9. PMID 18398601. S2CID 32820765.
- ^ Rang HP (2003). Pharmacology. Edinburgh: Churchill Livingstone. p. 187. ISBN 978-0-443-07145-4.
- ^ de Wit R, Aapro M, Blower PR (September 2005). «Is there a pharmacological basis for differences in 5-HT3-receptor antagonist efficacy in refractory patients?». Cancer Chemotherapy and Pharmacology. 56 (3): 231–238. doi:10.1007/s00280-005-1033-0. PMID 15838653. S2CID 27576150.
- ^ Frost M, Andersen TE, Yadav V, et al. (March 2010). «Patients with high-bone-mass phenotype owing to Lrp5-T253I mutation have low plasma levels of serotonin». Journal of Bone and Mineral Research. 25 (3): 673–675. doi:10.1002/jbmr.44. PMID 20200960. S2CID 24280062.
- ^ Rosen CJ (February 2009). «Breaking into bone biology: serotonin’s secrets». Nature Medicine. 15 (2): 145–146. doi:10.1038/nm0209-145. PMID 19197289. S2CID 5489589.
- ^ Mödder UI, Achenbach SJ, Amin S, et al. (February 2010). «Relation of serum serotonin levels to bone density and structural parameters in women». Journal of Bone and Mineral Research. 25 (2): 415–422. doi:10.1359/jbmr.090721. PMC 3153390. PMID 19594297.
- ^ Frost M, Andersen T, Gossiel F, et al. (August 2011). «Levels of serotonin, sclerostin, bone turnover markers as well as bone density and microarchitecture in patients with high-bone-mass phenotype due to a mutation in Lrp5». Journal of Bone and Mineral Research. 26 (8): 1721–1728. doi:10.1002/jbmr.376. PMID 21351148.
- ^ Kode A, Mosialou I, Silva BC, et al. (October 2012). «FOXO1 orchestrates the bone-suppressing function of gut-derived serotonin». The Journal of Clinical Investigation. 122 (10): 3490–3503. doi:10.1172/JCI64906. PMC 3461930. PMID 22945629.
- ^ Yadav VK, Balaji S, Suresh PS, et al. (March 2010). «Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis». Nature Medicine. 16 (3): 308–312. doi:10.1038/nm.2098. PMC 2836724. PMID 20139991.
- ^ Sugisawa E, Takayama Y, Takemura N, et al. (July 2019). «RNA Sensing by Gut Piezo1 Is Essential for Systemic Serotonin Synthesis». Cell. 182 (3): 609–624. doi:10.1016/j.cell.2020.06.022. PMID 32640190.
- ^ Ozanne SE, Hales CN (January 2004). «Lifespan: catch-up growth and obesity in male mice». Nature. 427 (6973): 411–412. Bibcode:2004Natur.427..411O. doi:10.1038/427411b. PMID 14749819. S2CID 40256021.
- ^ Lewis DS, Bertrand HA, McMahan CA, et al. (October 1986). «Preweaning food intake influences the adiposity of young adult baboons». J. Clin. Invest. 78 (4): 899–905. doi:10.1172/JCI112678. PMC 423712. PMID 3760191.
- ^ Hahn P (July 1984). «Effect of litter size on plasma cholesterol and insulin and some liver and adipose tissue enzymes in adult rodents». J. Nutr. 114 (7): 1231–1234. doi:10.1093/jn/114.7.1231. PMID 6376732.
- ^ Popa D, Léna C, Alexandre C, Adrien J (April 2008). «Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior». The Journal of Neuroscience. 28 (14): 3546–3554. doi:10.1523/JNEUROSCI.4006-07.2008. PMC 6671102. PMID 18385313.
- ^ Maciag D, Simpson KL, Coppinger D, et al. (January 2006). «Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry». Neuropsychopharmacology. 31 (1): 47–57. doi:10.1038/sj.npp.1300823. PMC 3118509. PMID 16012532.
- ^ Maciag D, Williams L, Coppinger D, Paul IA (February 2006). «Neonatal citalopram exposure produces lasting changes in behavior which are reversed by adult imipramine treatment». European Journal of Pharmacology. 532 (3): 265–269. doi:10.1016/j.ejphar.2005.12.081. PMC 2921633. PMID 16483567.
- ^ Holden C (October 2004). «Neuroscience. Prozac treatment of newborn mice raises anxiety». Science. 306 (5697): 792. doi:10.1126/science.306.5697.792. PMID 15514122.
- ^ Ansorge MS, Zhou M, Lira A, et al. (October 2004). «Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice». Science. 306 (5697): 879–881. Bibcode:2004Sci…306..879A. doi:10.1126/science.1101678. PMID 15514160.
- ^ Lesurtel M, Graf R, Aleil B, et al. (April 2006). «Platelet-derived serotonin mediates liver regeneration». Science. 312 (5770): 104–107. Bibcode:2006Sci…312..104L. doi:10.1126/science.1123842. PMID 16601191. S2CID 43189753.
- ^ Matondo RB, Punt C, Homberg J, et al. (April 2009). «Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration». American Journal of Physiology. Gastrointestinal and Liver Physiology. 296 (4): G963–G968. doi:10.1152/ajpgi.90709.2008. PMID 19246633.
- ^ Collet C, Schiltz C, Geoffroy V, et al. (February 2008). «The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation». FASEB Journal. 22 (2): 418–427. doi:10.1096/fj.07-9209com. PMC 5409955. PMID 17846081.
- ^ Yadav VK, Ryu JH, Suda N, et al. (November 2008). «Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum». Cell. 135 (5): 825–837. doi:10.1016/j.cell.2008.09.059. PMC 2614332. PMID 19041748.
- «It Takes Guts To Build Bone, Scientists Discover». ScienceDaily (Press release). 1 December 2008.
- ^ McDuffie JE, Motley ED, Limbird LE, Maleque MA (March 2000). «5-hydroxytryptamine stimulates phosphorylation of p44/p42 mitogen-activated protein kinase activation in bovine aortic endothelial cell cultures». Journal of Cardiovascular Pharmacology. 35 (3): 398–402. doi:10.1097/00005344-200003000-00008. PMID 10710124.
- ^ Marieb EN (2009). Essentials of Human Anatomy & Physiology (Eighth ed.). San Francisco: Pearson/Benjamin Cummings. p. 336. ISBN 978-0-321-51342-7.
- ^ Chang W, Kanda H, Ikeda R, Ling J, DeBerry JJ, Gu JG (September 2016). «Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals». Proceedings of the National Academy of Sciences of the United States of America. 113 (37): E5491-500. Bibcode:2016PNAS..113E5491C. doi:10.1073/pnas.1610176113. PMC 5027443. PMID 27573850.
- ^ Noguchi M, Furukawa KT, Morimoto M (December 2020). «Pulmonary neuroendocrine cells: physiology, tissue homeostasis and disease». Disease Models & Mechanisms. 13 (12): dmm046920. doi:10.1242/dmm.046920. PMC 7774893. PMID 33355253.
- ^ Fuller RW (1980). «Pharmacology of central serotonin neurons». Annual Review of Pharmacology and Toxicology. 20: 111–127. doi:10.1146/annurev.pa.20.040180.000551. PMID 6992697.
- ^ Titeler M, Lyon RA, Glennon RA (1988). «Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens». Psychopharmacology. 94 (2): 213–216. doi:10.1007/BF00176847. PMID 3127847. S2CID 24179554.
- ^ Nichols DE (2000). «Role of serotonergic neurons and 5-HT receptors in the action of hallucinogens». In Baumgarten HG, Gothert M (eds.). Serotoninergic Neurons and 5-HT Receptors in the CNS. Santa Clara, CA: Springer-Verlag TELOS. ISBN 978-3-540-66715-5.
- ^ Kapur S, Seeman P (2002). «NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia». Molecular Psychiatry. 7 (8): 837–844. doi:10.1038/sj.mp.4001093. PMID 12232776.
- ^ Johnson MP, Hoffman AJ, Nichols DE (December 1986). «Effects of the enantiomers of MDA, MDMA and related analogues on [3H]serotonin and [3H]dopamine release from superfused rat brain slices». European Journal of Pharmacology. 132 (2–3): 269–276. doi:10.1016/0014-2999(86)90615-1. PMID 2880735.
- ^ Goodman LS, Brunton LL, Chabner B, Knollmann BC (2001). Goodman and Gilman’s pharmacological basis of therapeutics. New York: McGraw-Hill. pp. 459–461. ISBN 978-0-07-162442-8.
- ^ Benmansour S, Cecchi M, Morilak DA, et al. (December 1999). «Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level». The Journal of Neuroscience. 19 (23): 10494–10501. doi:10.1523/JNEUROSCI.19-23-10494.1999. PMC 6782424. PMID 10575045.
- ^ Beitchman JH, Baldassarra L, Mik H, et al. (June 2006). «Serotonin transporter polymorphisms and persistent, pervasive childhood aggression». The American Journal of Psychiatry. 163 (6): 1103–1105. doi:10.1176/appi.ajp.163.6.1103. PMID 16741214.
- ^ Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. (June 2005). «5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression». Nature Neuroscience. 8 (6): 828–834. doi:10.1038/nn1463. PMID 15880108. S2CID 1864631.
- ^ Schinka JA, Busch RM, Robichaux-Keene N (February 2004). «A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety». Molecular Psychiatry. 9 (2): 197–202. doi:10.1038/sj.mp.4001405. PMID 14966478.
- ^ a b New AM, Nelson S, Leung JG (1 October 2015). Alexander E, Susla GM (eds.). «Psychiatric Emergencies in the Intensive Care Unit». AACN Advanced Critical Care. 26 (4): 285–293, quiz 294–295. doi:10.4037/NCI.0000000000000104. PMID 26484986.
- ^ Isbister GK, Bowe SJ, Dawson A, Whyte IM (2004). «Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose». Journal of Toxicology. Clinical Toxicology. 42 (3): 277–285. doi:10.1081/CLT-120037428. PMID 15362595. S2CID 43121327.
- ^ Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM (September 2003). «The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity». QJM. 96 (9): 635–642. doi:10.1093/qjmed/hcg109. PMID 12925718.
- ^ Frank C (July 2008). «Recognition and treatment of serotonin syndrome». Canadian Family Physician. 54 (7): 988–992. PMC 2464814. PMID 18625822.
- ^ Boyer EW, Shannon M (March 2005). «The serotonin syndrome». The New England Journal of Medicine. 352 (11): 1112–1120. doi:10.1056/NEJMra041867. PMID 15784664.
- ^ a b Baskin SI (1991). Principles of cardiac toxicology. Boca Raton: CRC Press. ISBN 978-0-8493-8809-5. Retrieved 3 February 2010.
- ^ Jähnichen S, Horowski R, Pertz H. «Pergolide and Cabergoline But not Lisuride Exhibit Agonist Efficacy at Serotonin 5-HT2B Receptors» (PDF). Retrieved 3 February 2010.
- ^ Adverse Drug Reactions Advisory Committee, Australia (2004). «Cardiac valvulopathy with pergolide». Aust Adv Drug React Bull. 23 (4). Archived from the original on 27 June 2012.
- ^ Schade R, Andersohn F, Suissa S, et al. (January 2007). «Dopamine agonists and the risk of cardiac-valve regurgitation». The New England Journal of Medicine. 356 (1): 29–38. doi:10.1056/NEJMoa062222. PMID 17202453.
- ^ Zanettini R, Antonini A, Gatto G, et al. (January 2007). «Valvular heart disease and the use of dopamine agonists for Parkinson’s disease». The New England Journal of Medicine. 356 (1): 39–46. doi:10.1056/NEJMoa054830. PMID 17202454.
- ^ «Food and Drug Administration Public Health Advisory». Food and Drug Administration. 29 March 2007. Retrieved 7 February 2010.
- ^ «MedWatch – 2007 Safety Information Alerts. Permax (pergolide) and generic equivalents». United States Food and Drug Administration. 29 March 2007. Retrieved 30 March 2007.
- ^ Tyler VE (September 1958). «Occurrence of serotonin in a hallucinogenic mushroom». Science. 128 (3326): 718. Bibcode:1958Sci…128..718T. doi:10.1126/science.128.3326.718. PMID 13580242.
- ^ Johnson DJ, Sanderson H, Brain RA, et al. (May 2007). «Toxicity and hazard of selective serotonin reuptake inhibitor antidepressants fluoxetine, fluvoxamine, and sertraline to algae». Ecotoxicology and Environmental Safety. 67 (1): 128–139. doi:10.1016/j.ecoenv.2006.03.016. PMID 16753215.
- ^ McGowan K, Guerina V, Wicks J, Donowitz M (1985). «Secretory Hormones of Entamoeba histolytica». Secretory hormones of Entamoeba histolytica. Ciba Foundation Symposium. Novartis Foundation Symposia. Vol. 112. pp. 139–154. doi:10.1002/9780470720936.ch8. ISBN 978-0470720936. PMID 2861068.
- ^ Banu N, Zaidi KR, Mehdi G, Mansoor T (July 2005). «Neurohumoral alterations and their role in amoebiasis». Indian Journal of Clinical Biochemistry. 20 (2): 142–145. doi:10.1007/BF02867414. PMC 3453840. PMID 23105547.
- ^ Acharya DP, Sen MR, Sen PC (August 1989). «Effect of exogenous 5-hydroxytryptamine on pathogenicity of Entamoeba histolytica in experimental animals». Indian Journal of Experimental Biology. 27 (8): 718–720. PMID 2561282.
- ^ Schröder P, Abele C, Gohr P, Stuhlfauth-Roisch U, Grosse W (1999). «Latest on enzymology of serotonin biosynthesis in walnut seeds». Tryptophan, Serotonin, and Melatonin. Advances in Experimental Medicine and Biology. Vol. 467. pp. 637–644. doi:10.1007/978-1-4615-4709-9_81. ISBN 978-0-306-46204-7. PMID 10721112.
- ^ Pénez N, Culioli G, Pérez T, et al. (October 2011). «Antifouling properties of simple indole and purine alkaloids from the Mediterranean gorgonian Paramuricea clavata«. Journal of Natural Products. 74 (10): 2304–2308. doi:10.1021/np200537v. PMID 21939218.
- ^ Guillén-Casla V, Rosales-Conrado N, León-González ME, et al. (April 2012). «Determination of serotonin and its precursors in chocolate samples by capillary liquid chromatography with mass spectrometry detection». Journal of Chromatography A. 1232: 158–165. doi:10.1016/j.chroma.2011.11.037. PMID 22186492.
- ^ Pelagio-Flores R, Ortíz-Castro R, Méndez-Bravo A, Macías-Rodríguez L, López-Bucio J (March 2011). «Serotonin, a tryptophan-derived signal conserved in plants and animals, regulates root system architecture probably acting as a natural auxin inhibitor in Arabidopsis thaliana». Plant & Cell Physiology. Oxford University Press (OUP). 52 (3): 490–508. doi:10.1093/pcp/pcr006. PMID 21252298.
- ^ a b Powell JJ, Carere J, Fitzgerald TL, Stiller J, Covarelli L, Xu Q, et al. (March 2017). «The Fusarium crown rot pathogen Fusarium pseudograminearum triggers a suite of transcriptional and metabolic changes in bread wheat (Triticum aestivum L.)». Annals of Botany. Oxford University Press (OUP). 119 (5): 853–867. doi:10.1093/aob/mcw207. PMC 5604588. PMID 27941094. S2CID 3823345.
- ^ Du Fall LA, Solomon PS (October 2013). «The necrotrophic effector SnToxA induces the synthesis of a novel phytoalexin in wheat». The New Phytologist. Wiley. 200 (1): 185–200. doi:10.1111/nph.12356. PMID 23782173.
- ^ Pasquet JC, Chaouch S, Macadré C, Balzergue S, Huguet S, Martin-Magniette ML, et al. (July 2014). «Differential gene expression and metabolomic analyses of Brachypodium distachyon infected by deoxynivalenol producing and non-producing strains of Fusarium graminearum». BMC Genomics. BioMed Central. 15 (1): 629. doi:10.1186/1471-2164-15-629. PMC 4124148. PMID 25063396.
- ^ Zeiss DR, Piater LA, Dubery IA (2021). «Hydroxycinnamate Amides: Intriguing Conjugates of Plant Protective Metabolites». Trends in Plant Science. Cell Press. 26 (2): 184–195. doi:10.1016/j.tplants.2020.09.011. ISSN 1360-1385. PMID 33036915. S2CID 222256660.
- ^ Jonz MG, EkateriniMercier A, JoffrePotter JW (2001). «Effects Of 5-HT (Serotonin) On Reproductive Behaviour In Heterodera Schachtii (Nematoda)». Canadian Journal of Zoology. 79 (9): 1727. doi:10.1139/z01-135.
- ^ Sawin ER, Ranganathan R, Horvitz HR (June 2000). «C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway». Neuron. 26 (3): 619–631. doi:10.1016/S0896-6273(00)81199-X. PMID 10896158. S2CID 9247380.
- ^ Niacaris T, Avery L (January 2003). «Serotonin regulates repolarization of the C. elegans pharyngeal muscle». The Journal of Experimental Biology. 206 (Pt 2): 223–231. doi:10.1242/jeb.00101. PMC 4441752. PMID 12477893.
- ^ Rosso MN, Jones JT, Abad P (2009). «RNAi and functional genomics in plant parasitic nematodes». Annual Review of Phytopathology. Annual Reviews. 47 (1): 207–232. doi:10.1146/annurev.phyto.112408.132605. PMID 19400649. p. 218:
Octopamine and serotonin regulates the activity of the M3 neurons that direct contraction of the pharynx during C. elegans feeding… Soaking Meloidogyne J2 in dsRNA in the presence of … resorcinol plus serotonin resulted in uptake of solutions and silencing of genes expressed in the intestine and esophageal glands.
- ^ Yeh SR, Fricke RA, Edwards DH (January 1996). «The effect of social experience on serotonergic modulation of the escape circuit of crayfish» (PDF). Science. 271 (5247): 366–369. Bibcode:1996Sci…271..366Y. CiteSeerX 10.1.1.470.6528. doi:10.1126/science.271.5247.366. PMID 8553075. S2CID 1575533.
- ^ Fry BG, Roelants K, Champagne DE, Scheib H, Tyndall JD, King GF, et al. (2009). «The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms». Annual Review of Genomics and Human Genetics. Annual Reviews. 10 (1): 483–511. doi:10.1146/annurev.genom.9.081307.164356. PMID 19640225.
- ^ «Serotonin, serotonin receptors and their actions in insects». Neurotransmitter. 2: 1–14. 2015. doi:10.14800/nt.314.
- ^ a b c Schoofs L, De Loof A, Van Hiel MB (31 January 2017). «Neuropeptides as Regulators of Behavior in Insects». Annual Review of Entomology. Annual Reviews. 62 (1): 35–52. doi:10.1146/annurev-ento-031616-035500. ISSN 0066-4170. PMID 27813667.
- ^ a b Wang X, Kang L (7 January 2014). «Molecular mechanisms of phase change in locusts». Annual Review of Entomology. Annual Reviews. 59 (1): 225–244. doi:10.1146/annurev-ento-011613-162019. PMID 24160426.
p. 231,
The change in the number of several potential neurotransmitters … such as serotonin… may play an important role in remodeling the CNS during phase change (26, 56, 80).
p. 233,
In the locust S. gregaria, the amount of serotonin in the thoracic ganglia was positively correlated with the extent of gregarious behavior induced by different periods of crowding. A series of pharmacological and behavioral experiments demonstrated that serotonin plays a key role in inducing initial behavioral gregarization (2, 80). However, serotonin is not responsible for maintaining gregarious behavior because its amount in long-term gregarious locusts is less than half that in long-term solitarious locusts (80). In L. migratoria, the injection of serotonin can also slightly initiate gregarious behavior, but serotonin when accompanying crowding treatment induced more solitarious-like behavior than did serotonin injection alone (48). Significant differences in serotonin levels were not found in brain tissues between the two phases of L. migratoria. A recent report by Tanaka & Nishide (97) measured attraction/avoidance behavior in S. gregaria after single and multiple injections of serotonin at different concentrations. Serotonin had only a short-term effect on the level of some locomotor activities and was not involved in the control of gregarious behavior (97). In addition, it is not clear how the neurotransmitter influences this unique behavior, because a binary logistic regression model used in these studies for the behavioral assay focused mostly on only one behavioral parameter representing an overall phase state. Obviously, behavioral phase change might involve alternative regulatory mechanisms in different locust species. Therefore, these studies demonstrate that CNS regulatory mechanisms governing initiation and maintenance of phase change are species specific and involve the interactions between these neurotransmitters.
Given the key roles of aminergic signaling, what are the downstream pathways involved in the establishment of long-term memory? Ott et al. (63) investigated the role of [] protein kinase[] in the phase change in S. gregaria: … cAMP-dependent protein kinase A (PKA). Through use of pharmacological and RNAi intervention, these authors have demonstrated that PKA… has a critical role in modulating the propensity of locusts to acquire and express gregarious behavior. … Unfortunately, although a correlation between serotonin and PKA was hypothesized, direct evidence was not provided. - ^ a b Zhang L, Lecoq M, Latchininsky A, Hunter D (January 2019). «Locust and Grasshopper Management». Annual Review of Entomology. Annual Reviews. 64 (1): 15–34. doi:10.1146/annurev-ento-011118-112500. PMID 30256665. S2CID 52843907. p. 20:
…gregarization is evoked by… tactile stimulation… Tactile stimuli trigger the increase of biogenic amines, particularly serotonin, in the locust nervous system (1, 116); these amines play critical roles in the neurophysiology of locust behavioral phase change.
- ^ a b Anstey ML, Rogers SM, Ott SR, Burrows M, Simpson SJ (January 2009). «Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts». Science. 323 (5914): 627–630. Bibcode:2009Sci…323..627A. doi:10.1126/science.1165939. PMID 19179529. S2CID 5448884.
- Morgan J (29 January 2009). «Locust swarms ‘high’ on serotonin». BBC News.
- ^ Sitaraman D, LaFerriere H, Birman S, Zars T (June 2012). «Serotonin is critical for rewarded olfactory short-term memory in Drosophila». Journal of Neurogenetics. 26 (2): 238–244. doi:10.3109/01677063.2012.666298. PMID 22436011. S2CID 23639918.
- ^ Bicker G, Menzel R (January 1989). «Chemical codes for the control of behaviour in arthropods». Nature. 337 (6202): 33–39. Bibcode:1989Natur.337…33B. doi:10.1038/337033a0. PMID 2562906. S2CID 223750.
- ^ Cai M, Li Z, Fan F, Huang Q, Shao X, Song G (March 2010). «Design and synthesis of novel insecticides based on the serotonergic ligand 1-[(4-aminophenyl)ethyl]-4-[3-(trifluoromethyl)phenyl]piperazine (PAPP)». Journal of Agricultural and Food Chemistry. 58 (5): 2624–2629. doi:10.1021/jf902640u. PMID 20000410.
- ^ Manahan SE (2002). Toxicological Chemistry and Biochemistry (3rd ed.). CRC Press. p. 393. ISBN 978-1-4200-3212-3.
- ^ Postma TL (2009). «Neurotoxic Animal Poisons and Venoms». In Dobbs MR (ed.). Clinical Neurotoxicology. W.B. Saunders. pp. 463–489. doi:10.1016/B978-032305260-3.50049-6. ISBN 978-0-323-05260-3.
- ^ Gadenne C, Barrozo RB, Anton S (11 March 2016). «Plasticity in Insect Olfaction: To Smell or Not to Smell?». Annual Review of Entomology. Annual Reviews. 61 (1): 317–333. doi:10.1146/annurev-ento-010715-023523. hdl:11336/19586. PMID 26982441. S2CID 207568844.
- ^ Dierick HA, Greenspan RJ (May 2007). «Serotonin and neuropeptide F have opposite modulatory effects on fly aggression». Nature Genetics. 39 (5): 678–682. doi:10.1038/ng2029. PMID 17450142. S2CID 33768246.
- ^ Stoffolano JG, Haselton AT (7 January 2013). «The adult Dipteran crop: a unique and overlooked organ». Annual Review of Entomology. Annual Reviews. 58 (1): 205–225. doi:10.1146/annurev-ento-120811-153653. PMID 23317042.
- ^ a b c d e f Bacqué-Cazenave J, Bharatiya R, Barrière G, Delbecque JP, Bouguiyoud N, Di Giovanni G, et al. (February 2020). «Serotonin in Animal Cognition and Behavior». International Journal of Molecular Sciences. 21 (5): 1649. doi:10.3390/ijms21051649. PMC 7084567. PMID 32121267.
- ^ a b c d e Lucki I (August 1998). «The spectrum of behaviors influenced by serotonin». Biological Psychiatry. 44 (3): 151–162. doi:10.1016/s0006-3223(98)00139-5. PMID 9693387. S2CID 3001666.
- ^ a b c Backström T, Winberg S (25 October 2017). «Serotonin Coordinates Responses to Social Stress-What We Can Learn from Fish». Frontiers in Neuroscience. 11: 595. doi:10.3389/fnins.2017.00595. PMC 5669303. PMID 29163002.
- ^ Berger M, Gray JA, Roth BL (1 February 2009). «The expanded biology of serotonin». Annual Review of Medicine. 60 (1): 355–366. doi:10.1146/annurev.med.60.042307.110802. PMC 5864293. PMID 19630576.
- ^ Alberghina D, Piccione G, Pumilia G, Gioè M, Rizzo M, Raffo P, Panzera M (July 2019). «Daily fluctuation of urine serotonin and cortisol in healthy shelter dogs and influence of intraspecific social exposure». Physiology & Behavior. 206: 1–6. doi:10.1016/j.physbeh.2019.03.016. PMID 30898540. S2CID 81965422.
- ^ Riggio G, Mariti C, Sergi V, Diverio S, Gazzano A (December 2020). «Serotonin and Tryptophan Serum Concentrations in Shelter Dogs Showing Different Behavioural Responses to a Potentially Stressful Procedure». Veterinary Sciences. 8 (1): 1. doi:10.3390/vetsci8010001. PMC 7824451. PMID 33374183.
- ^ Hydbring-Sandberg E, Larsson E, Madej A, Höglund OV (July 2021). «Short-term effect of ovariohysterectomy on urine serotonin, cortisol, testosterone and progesterone in bitches». BMC Research Notes. 14 (1): 265. doi:10.1186/s13104-021-05680-y. PMC 8272283. PMID 34246304.
- ^ a b c Winberg S, Thörnqvist PO (June 2016). «Role of brain serotonin in modulating fish behavior». Current Zoology. 62 (3): 317–323. doi:10.1093/cz/zow037. PMC 5804243. PMID 29491919.
- ^ a b c d Olivier B, Mos J, van der Heyden J, Hartog J (1 February 1989). «Serotonergic modulation of social interactions in isolated male mice». Psychopharmacology. 97 (2): 154–156. doi:10.1007/BF00442239. PMID 2498921. S2CID 37170174.
- ^ Huber R, Smith K, Delago A, Isaksson K, Kravitz EA (May 1997). «Serotonin and aggressive motivation in crustaceans: altering the decision to retreat». Proceedings of the National Academy of Sciences of the United States of America. 94 (11): 5939–5942. Bibcode:1997PNAS…94.5939H. doi:10.1073/pnas.94.11.5939. PMC 20885. PMID 9159179.
- ^ Sanchez CL, Biskup CS, Herpertz S, Gaber TJ, Kuhn CM, Hood SH, Zepf FD (May 2015). «The Role of Serotonin (5-HT) in Behavioral Control: Findings from Animal Research and Clinical Implications». The International Journal of Neuropsychopharmacology. 18 (10): pyv050. doi:10.1093/ijnp/pyv050. PMC 4648158. PMID 25991656.
- ^ a b c d Petersen CL, Hurley LM (October 2017). «Putting it in Context: Linking Auditory Processing with Social Behavior Circuits in the Vertebrate Brain». Integrative and Comparative Biology. 57 (4): 865–877. doi:10.1093/icb/icx055. PMC 6251620. PMID 28985384.
- ^ Loer CM, Kenyon CJ (December 1993). «Serotonin-deficient mutants and male mating behavior in the nematode Caenorhabditis elegans». The Journal of Neuroscience. 13 (12): 5407–5417. doi:10.1523/JNEUROSCI.13-12-05407.1993. PMC 6576401. PMID 8254383.
- ^ Lipton J, Kleemann G, Ghosh R, et al. (August 2004). «Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate». The Journal of Neuroscience. 24 (34): 7427–7434. doi:10.1523/JNEUROSCI.1746-04.2004. PMC 6729642. PMID 15329389.
- ^ a b Murakami H, Murakami S (August 2007). «Serotonin receptors antagonistically modulate Caenorhabditis elegans longevity». Aging Cell. 6 (4): 483–488. doi:10.1111/j.1474-9726.2007.00303.x. PMID 17559503. S2CID 8345654.
- ^ Kaplan DD, Zimmermann G, Suyama K, et al. (July 2008). «A nucleostemin family GTPase, NS3, acts in serotonergic neurons to regulate insulin signaling and control body size». Genes & Development. 22 (14): 1877–1893. doi:10.1101/gad.1670508. PMC 2492735. PMID 18628395.
- ^ Ruaud AF, Thummel CS (July 2008). «Serotonin and insulin signaling team up to control growth in Drosophila». Genes & Development. 22 (14): 1851–1855. doi:10.1101/gad.1700708. PMC 2735276. PMID 18628391.
- ^ Davidson S, Prokonov D, Taler M, et al. (February 2009). «Effect of exposure to selective serotonin reuptake inhibitors in utero on fetal growth: potential role for the IGF-I and HPA axes». Pediatric Research. 65 (2): 236–241. doi:10.1203/PDR.0b013e318193594a. PMID 19262294.
- ^ Ben Arous J, Laffont S, Chatenay D (October 2009). Brezina V (ed.). «Molecular and sensory basis of a food related two-state behavior in C. elegans». PLOS ONE. 4 (10): e7584. Bibcode:2009PLoSO…4.7584B. doi:10.1371/journal.pone.0007584. PMC 2762077. PMID 19851507.
- ^ Sze JY, Victor M, Loer C, Shi Y, Ruvkun G (February 2000). «Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant». Nature. 403 (6769): 560–564. Bibcode:2000Natur.403..560S. doi:10.1038/35000609. PMID 10676966. S2CID 4394553.
- ^ Murakami H, Bessinger K, Hellmann J, Murakami S (July 2008). «Manipulation of serotonin signal suppresses early phase of behavioral aging in Caenorhabditis elegans». Neurobiology of Aging. 29 (7): 1093–1100. doi:10.1016/j.neurobiolaging.2007.01.013. PMID 17336425. S2CID 37671716.
- ^ Côté F, Thévenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, et al. (November 2003). «Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function». Proceedings of the National Academy of Sciences of the United States of America. 100 (23): 13525–13530. Bibcode:2003PNAS..10013525C. doi:10.1073/pnas.2233056100. PMC 263847. PMID 14597720.
- ^ Alarcón J, Cid E, Lillo L, Céspedesa C, Aguila S, Alderete JB (2008). «Biotransformation of indole derivatives by mycelial cultures». Zeitschrift für Naturforschung C. 63 (1–2): 82–84. doi:10.1515/znc-2008-1-215. PMID 18386493. S2CID 29472174.
- ^ Sismaet HJ, Goluch ED (June 2018). «Electrochemical Probes of Microbial Community Behavior». Annual Review of Analytical Chemistry. Annual Reviews. 11 (1): 441–461. Bibcode:2018ARAC…11..441S. doi:10.1146/annurev-anchem-061417-125627. PMID 29490192. S2CID 3632265. p. 449:
Table 1 The respective potential peaks for various electroactive biomolecules that are produced or consumed by microbes reported in the literaturea … Serotonin | Indium tin oxide | +0.67 | 66
- ^ Henson JM, Butler MJ, Day AW (1999). «The Dark Side of the Mycelium: Melanins of Phytopathogenic Fungi». Annual Review of Phytopathology. Annual Reviews. 37 (1): 447–471. doi:10.1146/annurev.phyto.37.1.447. PMID 11701831.
- ^ Anthony M (1984). «Serotonin antagonists». Aust N Z J Med. 14 (6): 888–895. doi:10.1111/j.1445-5994.1984.tb03802.x. PMID 6398056. S2CID 28327178.
- ^ Erspamer V (1954). «Pharmacology of indole-alkylamines». Pharmacol Rev. 6 (4): 425–487. PMID 13236482.
- ^ Negri L (2006). «[Vittorio Erspamer (1909–1999)]». Medicina Nei Secoli. 18 (1): 97–113. PMID 17526278.
- ^ Rapport MM, Green AA, Page IH (December 1948). «Serum vasoconstrictor, serotonin; isolation and characterization». The Journal of Biological Chemistry. 176 (3): 1243–1251. doi:10.1016/S0021-9258(18)57137-4. PMID 18100415.
- ^ Feldberg W, Toh CC (February 1953). «Distribution of 5-hydroxytryptamine (serotonin, enteramine) in the wall of the digestive tract». The Journal of Physiology. 119 (2–3): 352–362. doi:10.1113/jphysiol.1953.sp004850. PMC 1392800. PMID 13035756.
- ^ SciFinder – Serotonin Substance Detail. Accessed (4 November 2012).[full citation needed]
- ^ Twarog BM, Page IH (October 1953). «Serotonin content of some mammalian tissues and urine and a method for its determination». The American Journal of Physiology. 175 (1): 157–161. doi:10.1152/ajplegacy.1953.175.1.157. PMID 13114371.
- ^ Page IH (1 July 1954). «Serotonin (5-Hydroxytryptamine)». Physiological Reviews. American Physiological Society. 34 (3): 563–588. doi:10.1152/physrev.1954.34.3.563. ISSN 0031-9333. PMID 13185755.
Further reading[edit]
- Gutknecht L, Jacob C, Strobel A, Kriegebaum C, Müller J, Zeng Y, et al. (June 2007). «Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation». The International Journal of Neuropsychopharmacology. 10 (3): 309–320. doi:10.1017/S1461145706007437. PMID 17176492.
External links[edit]
Wikimedia Commons has media related to Serotonin.
- 5-Hydroxytryptamine MS Spectrum
- Serotonin bound to proteins in the PDB
- PsychoTropicalResearch Extensive reviews on serotonergic drugs and Serotonin Syndrome.
- Molecule of the Month: Serotonin at University of Bristol
- 60-Second Psych: No Fair! My Serotonin Level Is Low, Scientific American
- Serotonin Test Interpretation on ClinLab Navigator.
 |
|
| Clinical data | |
|---|---|
| Other names | 5-HT, 5-Hydroxytryptamine, Enteramine, Thrombocytin, 3-(β-Aminoethyl)-5-hydroxyindole, Thrombotonin |
| Physiological data | |
| Source tissues | raphe nuclei, enterochromaffin cells |
| Target tissues | system-wide |
| Receptors | 5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 |
| Agonists | Indirectly: SSRIs, MAOIs |
| Precursor | 5-HTP |
| Biosynthesis | Aromatic L-amino acid decarboxylase |
| Metabolism | MAO |
| Identifiers | |
|
IUPAC name
|
|
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| ChemSpider |
|
| KEGG |
|
| PDB ligand |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.000.054 |

|
|
| Names | |
|---|---|
| IUPAC name
5-Hydroxytryptamine |
|
| Preferred IUPAC name
3-(2-Aminoethyl)-1H-indol-5-ol |
|
| Other names
5-Hydroxytryptamine, 5-HT, Enteramine; Thrombocytin, 3-(β-Aminoethyl)-5-hydroxyindole, 3-(2-Aminoethyl)indol-5-ol, Thrombotonin |
|
| Identifiers | |
|
CAS Number |
|
|
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.000.054 |
|
IUPHAR/BPS |
|
| KEGG |
|
| MeSH | Serotonin |
|
PubChem CID |
|
| UNII |
|
|
CompTox Dashboard (EPA) |
|
|
InChI
|
|
|
SMILES
|
|
| Properties | |
|
Chemical formula |
C10H12N2O |
| Molar mass | 176.215 g/mol |
| Appearance | White powder |
| Melting point | 167.7 °C (333.9 °F; 440.8 K) 121–122 °C (ligroin)[3] |
| Boiling point | 416 ± 30 °C (at 760 Torr)[1] |
|
Solubility in water |
slightly soluble |
| Acidity (pKa) | 10.16 in water at 23.5 °C[2] |
|
Dipole moment |
2.98 D |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose) |
750 mg/kg (subcutaneous, rat),[4] 4500 mg/kg (intraperitoneal, rat),[5] 60 mg/kg (oral, rat) |
| Safety data sheet (SDS) | External MSDS |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references |
Serotonin ([6][7][8]) or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction.[9] Approximately 90% of the serotonin that the body produces is in the intestinal tract.[10]
Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin.[11] Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is released during agitation and vasoconstriction, where it then acts as an agonist to other platelets.[12]
Approximately 90% of the human body’s total serotonin is located in the enterochromaffin cells in the GI tract, where it regulates intestinal movements.[13][14] About 8% is found in platelets and 1–2% in the CNS.[15] The serotonin is secreted luminally and basolaterally, which leads to increased serotonin uptake by circulating platelets and activation after stimulation, which gives increased stimulation of myenteric neurons and gastrointestinal motility.[16] The remainder is synthesized in serotonergic neurons of the CNS, where it has various functions. These include the regulation of mood, appetite, and sleep. Serotonin also has some cognitive functions, including memory and learning.
Several classes of antidepressants, such as the SSRIs and the SNRIs among others, interfere with the normal reabsorption of serotonin after it is done with the transmission of the signal, therefore augmenting the neurotransmitter levels in the synapses.
Serotonin secreted from the enterochromaffin cells eventually finds its way out of tissues into the blood. There, it is actively taken up by blood platelets, which store it. When the platelets bind to a clot, they release serotonin, where it can serve as a vasoconstrictor or a vasodilator while regulating hemostasis and blood clotting. In high concentrations, serotonin acts as a vasoconstrictor by contracting endothelial smooth muscle directly or by potentiating the effects of other vasoconstrictors (e.g. angiotensin II, norepinephrine). The vasoconstrictive property is mostly seen in pathologic states affecting the endothelium – such as atherosclerosis or chronic hypertension. In physiologic states, vasodilation occurs through the serotonin mediated release of nitric oxide from endothelial cells. Additionally, it inhibits the release of norepinephrine from adrenergic nerves.[17] Serotonin is also a growth factor for some types of cells, which may give it a role in wound healing. There are various serotonin receptors.
Serotonin is metabolized mainly to 5-HIAA, chiefly by the liver. Metabolism involves first oxidation by monoamine oxidase to the corresponding aldehyde. The rate-limiting step is hydride transfer from serotonin to the flavin cofactor.[18] There follows oxidation by aldehyde dehydrogenase to 5-HIAA, the indole acetic-acid derivative. The latter is then excreted by the kidneys.
Besides mammals, serotonin is found in all bilateral animals including worms and insects,[19] as well as in fungi and in plants.[20] Serotonin’s presence in insect venoms and plant spines serves to cause pain, which is a side-effect of serotonin injection.[21][22] Serotonin is produced by pathogenic amoebae, and its effect in the human gut is diarrhea.[23] Its widespread presence in many seeds and fruits may serve to stimulate the digestive tract into expelling the seeds.[24]
Biological role[edit]
Serotonin is involved in numerous physiological processes, including sleep, thermoregulation, learning and memory, pain, (social) behavior,[25] sexual activity, feeding, motor activity, biological rhythms and possibly others.[26] In less complex animals, such as some invertebrates, serotonin regulates feeding and other processes.[27] In plants serotonin synthesis seems to be associated with stress signals.[20][28]
Cellular effects[edit]
Serotonin primarily acts through its receptors and its effects depend on which cells and tissues express these receptors (see below).[26]
Receptors[edit]
The 5-HT receptors, the receptors for serotonin, are located on the cell membrane of nerve cells and other cell types in animals, and mediate the effects of serotonin as the endogenous ligand and of a broad range of pharmaceutical and psychedelic drugs. Except for the 5-HT3 receptor, a ligand-gated ion channel, all other 5-HT receptors are G-protein-coupled receptors (also called seven-transmembrane, or heptahelical receptors) that activate an intracellular second messenger cascade.[29]
Termination[edit]
Serotonergic action is terminated primarily via uptake of 5-HT from the synapse. This is accomplished through the specific monoamine transporter for 5-HT, SERT, on the presynaptic neuron. Various agents can inhibit 5-HT reuptake, including cocaine, dextromethorphan (an antitussive), tricyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs). A 2006 study conducted by the University of Washington suggested that a newly discovered monoamine transporter, known as PMAT, may account for «a significant percentage of 5-HT clearance».[30]
Contrasting with the high-affinity SERT, the PMAT has been identified as a low-affinity transporter, with an apparent Km of 114 micromoles/l for serotonin; approximately 230 times higher than that of SERT. However, the PMAT, despite its relatively low serotonergic affinity, has a considerably higher transport ‘capacity’ than SERT, «resulting in roughly comparable uptake efficiencies to SERT in heterologous expression systems.»[30] The study also suggests some SSRIs, such as fluoxetine and sertraline anti-depressants, inhibit PMAT but at IC50 values which surpass the therapeutic plasma concentrations by up to four orders of magnitude. Therefore, SSRI monotherapy is «ineffective» in PMAT inhibition. At present, no known pharmaceuticals are known to appreciably inhibit PMAT at normal therapeutic doses. The PMAT also suggestively transports dopamine and norepinephrine, albeit at Km values even higher than that of 5-HT (330–15,000 μmoles/L).[30]
Serotonylation[edit]
Serotonin can also signal through a nonreceptor mechanism called serotonylation, in which serotonin modifies proteins.[31] This process underlies serotonin’s effects upon platelet-forming cells (thrombocytes) in which it links to the modification of signaling enzymes called GTPases that then trigger the release of vesicle contents by exocytosis.[32] A similar process underlies the pancreatic release of insulin.[31]
The effects of serotonin upon vascular smooth muscle tone – the biological function after which serotonin was originally named – depend upon the serotonylation of proteins involved in the contractile apparatus of muscle cells.[33]
| Receptor | Ki (nM)[34] | Receptor function[Note 1] |
|---|---|---|
| 5-HT1 receptor family signals via Gi/o inhibition of adenylyl cyclase. | ||
| 5-HT1A | 3.17 | Memory[vague] (agonists ↓); learning[vague] (agonists ↓); anxiety (agonists ↓); depression (agonists ↓); positive, negative, and cognitive symptoms of schizophrenia (partial agonists ↓); analgesia (agonists ↑); aggression (agonists ↓); dopamine release in the prefrontal cortex (agonists ↑); serotonin release and synthesis (agonists ↓) |
| 5-HT1B | 4.32 | Vasoconstriction (agonists ↑); aggression (agonists ↓); bone mass (↓). Serotonin autoreceptor. |
| 5-HT1D | 5.03 | Vasoconstriction (agonists ↑) |
| 5-HT1E | 7.53 | |
| 5-HT1F | 10 | |
| 5-HT2 receptor family signals via Gq activation of phospholipase C. | ||
| 5-HT2A | 11.55 | Psychedelia (agonists ↑); depression (agonists & antagonists ↓); anxiety (antagonists ↓); positive and negative symptoms of schizophrenia (antagonists ↓); norepinephrine release from the locus coeruleus (antagonists ↑); glutamate release in the prefrontal cortex (agonists ↑); dopamine in the prefrontal cortex (agonists ↑);[35] urinary bladder contractions (agonists ↑)[36] |
| 5-HT2B | 8.71 | Cardiovascular functioning (agonists increase risk of pulmonary hypertension), empathy (via von Economo neurons[37]) |
| 5-HT2C | 5.02 | Dopamine release into the mesocorticolimbic pathway (agonists ↓); acetylcholine release in the prefrontal cortex (agonists ↑); dopaminergic and noradrenergic activity in the frontal cortex (antagonists ↑);[38] appetite (agonists ↓); antipsychotic effects (agonists ↑); antidepressant effects (agonists & antagonists ↑) |
| Other 5-HT receptors | ||
| 5-HT3 | 593 | Emesis (agonists ↑); anxiolysis (antagonists ↑). |
| 5-HT4 | 125.89 | Movement of food across the GI tract (agonists ↑); memory & learning (agonists ↑); antidepressant effects (agonists ↑). Signalling via Gαs activation of adenylyl cyclase. |
| 5-HT5A | 251.2 | Memory consolidation.[39] Signals via Gi/o inhibition of adenylyl cyclase. |
| 5-HT6 | 98.41 | Cognition (antagonists ↑); antidepressant effects (agonists & antagonists ↑); anxiogenic effects (antagonists ↑[40]). Gs signalling via activating adenylyl cyclase. |
| 5-HT7 | 8.11 | Cognition (antagonists ↑); antidepressant effects (antagonists ↑). Acts by Gs signalling via activating adenylyl cyclase. |
Nervous system[edit]
The neurons of the raphe nuclei are the principal source of 5-HT release in the brain.[41] There are nine raphe nuclei, designated B1–B9, which contain the majority of serotonin-containing neurons (some scientists chose to group the nuclei raphes lineares into one nucleus), all of which are located along the midline of the brainstem, and centered on the reticular formation.[42][43] Axons from the neurons of the raphe nuclei form a neurotransmitter system reaching almost every part of the central nervous system. Axons of neurons in the lower raphe nuclei terminate in the cerebellum and spinal cord, while the axons of the higher nuclei spread out in the entire brain.
Ultrastructure and function[edit]
The serotonin nuclei may also be divided into two main groups, the rostral and caudal containing three and four nuclei respectively. The rostral group consists of the caudal linear nuclei (B8), the dorsal raphe nuclei (B6 and B7) and the median raphe nuclei (B5, B8 and B9), that project into multiple cortical and subcortical structures. The caudal group consists of the nucleus raphe magnus (B3), raphe obscurus nucleus (B2), raphe pallidus nucleus (B1), and lateral medullary reticular formation, that project into the brainstem.[44]
The serotonergic pathway is involved in sensorimotor function, with pathways projecting both into cortical (Dorsal and Median Raphe Nuclei), subcortical, and spinal areas involved in motor activity. Pharmacological manipulation suggests that serotonergic activity increases with motor activity while firing rates of serotonergic neurons increase with intense visual stimuli. Animal models suggest that kainate signaling negatively regulates serotonin actions in the retina, with possible implications for the control of the visual system.[45] The descending projections form a pathway that inhibits pain called the «descending inhibitory pathway» that may be relevant to a disorder such as fibromyalgia, migraine, and other pain disorders, and the efficacy of antidepressants in them.[46]
Serotonergic projections from the caudal nuclei are involved in regulating mood and emotion, and hypo-[47] or hyper-serotonergic[48] states may be involved in depression and sickness behavior.
Microanatomy[edit]
Serotonin is released into the synapse, or space between neurons, and diffuses over a relatively wide gap (>20 nm) to activate 5-HT receptors located on the dendrites, cell bodies, and presynaptic terminals of adjacent neurons.
When humans smell food, dopamine is released to increase the appetite. But, unlike in worms, serotonin does not increase anticipatory behaviour in humans; instead, the serotonin released while consuming activates 5-HT2C receptors on dopamine-producing cells. This halts their dopamine release, and thereby serotonin decreases appetite. Drugs that block 5-HT2C receptors make the body unable to recognize when it is no longer hungry or otherwise in need of nutrients, and are associated with weight gain,[49] especially in people with a low number of receptors.[50] The expression of 5-HT2C receptors in the hippocampus follows a diurnal rhythm,[51] just as the serotonin release in the ventromedial nucleus, which is characterised by a peak at morning when the motivation to eat is strongest.[52]
In macaques, alpha males have twice the level of serotonin in the brain as subordinate males and females (measured by the concentration of 5-HIAA in the cerebrospinal fluid (CSF)). Dominance status and CSF serotonin levels appear to be positively correlated. When dominant males were removed from such groups, subordinate males begin competing for dominance. Once new dominance hierarchies were established, serotonin levels of the new dominant individuals also increased to double those in subordinate males and females. The reason why serotonin levels are only high in dominant males, but not dominant females has not yet been established.[53]
In humans, levels of 5-HT1A receptor inhibition in the brain show negative correlation with aggression,[54] and a mutation in the gene that codes for the 5-HT2A receptor may double the risk of suicide for those with that genotype.[55] Serotonin in the brain is not usually degraded after use, but is collected by serotonergic neurons by serotonin transporters on their cell surfaces. Studies have revealed nearly 10% of total variance in anxiety-related personality depends on variations in the description of where, when and how many serotonin transporters the neurons should deploy.[56]
Psychological influences[edit]
Serotonin has been implicated in cognition, mood, anxiety and psychosis, but strong clarity has not been achieved.[57][58]
Autism spectrum disorder (ASD)[edit]
In regards to research for neurotransmitters and effects on patients with Autism Spectrum Disorder (ASD), 5-HT has been studied the most in terms of research efforts and investigations.[59] As noted, 5-HT signaling does facilitate many neural processes including that of neurogenesis, cell migration and survival, synaptogenesis, and synaptic plasticity.[59] It was noted that 45% of tested ASD subjects contained high levels of 5-HT in their blood.[59] In addition, investigations performed on ASD-like animal models reported that hyperserotonemia significantly reduced the motivation for social interest through inhibition of separation distress, which could be related in the ASD patients that have social impairments.[59]
Outside the nervous system[edit]
In the digestive tract (emetic)[edit]
Serotonin regulates gastrointestinal function. The gut is surrounded by enterochromaffin cells, which release serotonin in response to food in the lumen. This makes the gut contract around the food. Platelets in the veins draining the gut collect excess serotonin. There are often serotonin abnormalities in gastrointestinal disorders such as constipation and irritable bowel syndrome.[60]
If irritants are present in the food, the enterochromaffin cells release more serotonin to make the gut move faster, i.e., to cause diarrhea, so the gut is emptied of the noxious substance. If serotonin is released in the blood faster than the platelets can absorb it, the level of free serotonin in the blood is increased. This activates 5-HT3 receptors in the chemoreceptor trigger zone that stimulate vomiting.[61] Thus, drugs and toxins stimulate serotonin release from enterochromaffin cells in the gut wall. The enterochromaffin cells not only react to bad food but are also very sensitive to irradiation and cancer chemotherapy. Drugs that block 5HT3 are very effective in controlling the nausea and vomiting produced by cancer treatment, and are considered the gold standard for this purpose.[62]
Bone metabolism[edit]
In mice and humans, alterations in serotonin levels and signalling have been shown to regulate bone mass.[63][64][65][66] Mice that lack brain serotonin have osteopenia, while mice that lack gut serotonin have high bone density. In humans, increased blood serotonin levels have been shown to be significant negative predictor of low bone density. Serotonin can also be synthesized, albeit at very low levels, in the bone cells. It mediates its actions on bone cells using three different receptors. Through 5-HT1B receptors, it negatively regulates bone mass, while it does so positively through 5-HT2B receptors and 5-HT2C receptors. There is very delicate balance between physiological role of gut serotonin and its pathology. Increase in the extracellular content of serotonin results in a complex relay of signals in the osteoblasts culminating in FoxO1/ Creb and ATF4 dependent transcriptional events.[67] Following the 2008 findings that gut serotonin regulates bone mass, the mechanistic investigations into what regulates serotonin synthesis from the gut in the regulation of bone mass have started. Piezo1 has been shown to sense RNA in the gut and relay this information through serotonin synthesis to the bone by acting as a sensor of single-stranded RNA (ssRNA) governing 5-HT production. Intestinal epithelium-specific deletion of mouse Piezo1 profoundly disturbed gut peristalsis, impeded experimental colitis, and suppressed serum 5-HT levels. Because of systemic 5-HT deficiency, conditional knockout of Piezo1 increased bone formation. Notably, fecal ssRNA was identified as a natural Piezo1 ligand, and ssRNA-stimulated 5-HT synthesis from the gut was evoked in a MyD88/TRIF-independent manner. Colonic infusion of RNase A suppressed gut motility and increased bone mass. These findings suggest gut ssRNA as a master determinant of systemic 5-HT levels, indicating the ssRNA-Piezo1 axis as a potential prophylactic target for treatment of bone and gut disorders. Studies in 2008, 2010 and 2019 have opened the potential for serotonin research to treat bone mass disorders.[68][69]
Organ development[edit]
Since serotonin signals resource availability it is not surprising that it affects organ development. Many human and animal studies have shown that nutrition in early life can influence, in adulthood, such things as body fatness, blood lipids, blood pressure, atherosclerosis, behavior, learning, and longevity.[70][71][72] Rodent experiment shows that neonatal exposure to SSRIs makes persistent changes in the serotonergic transmission of the brain resulting in behavioral changes,[73][74] which are reversed by treatment with antidepressants.[75] By treating normal and knockout mice lacking the serotonin transporter with fluoxetine scientists showed that normal emotional reactions in adulthood, like a short latency to escape foot shocks and inclination to explore new environments were dependent on active serotonin transporters during the neonatal period.[76][77]
Human serotonin can also act as a growth factor directly. Liver damage increases cellular expression of 5-HT2A and 5-HT2B receptors, mediating liver compensatory regrowth (see Liver § Regeneration and transplantation)[78] Serotonin present in the blood then stimulates cellular growth to repair liver damage.[79]
5HT2B receptors also activate osteocytes, which build up bone[80] However, serotonin also inhibits osteoblasts, through 5-HT1B receptors.[81]
Cardiovascular growth factor[edit]
Serotonin, in addition, evokes endothelial nitric oxide synthase activation and stimulates, through a 5-HT1B receptor-mediated mechanism, the phosphorylation of p44/p42 mitogen-activated protein kinase activation in bovine aortic endothelial cell cultures.[clarification needed][82] In blood, serotonin is collected from plasma by platelets, which store it. It is thus active wherever platelets bind in damaged tissue, as a vasoconstrictor to stop bleeding, and also as a fibrocyte mitotic (growth factor), to aid healing.[83]
Skin[edit]
Serotonin is also produced by Merkel cells which are part of the somatosensory system.[84]
Lungs[edit]
Pulmonary neuroendocrine cells are specialized epithelial cells that occur as solitary cells or as clusters called neuroepithelial bodies in the lung. Pulmonary neuroendocrine cells are also known as Kulchitsky cells or K cells.[85]
Pharmacology[edit]
Several classes of drugs target the 5-HT system, including some antidepressants, antipsychotics, anxiolytics, antiemetics, and antimigraine drugs, as well as, the psychedelic drugs and empathogens.
Mechanism of action[edit]
At rest, serotonin is stored within the vesicles of presynaptic neurons. When stimulated by nerve impulses, serotonin is released as a neurotransmitter into the synapse, reversibly binding to the postsynaptic receptor to induce a nerve impulse on the postsynaptic neuron. Serotonin can also bind to auto-receptors on the presynaptic neuron to regulate the synthesis and release of serotonin. Normally serotonin is taken back into the presynaptic neuron to stop its action, then reused or broken down by monoamine oxidase.[86]
Psychedelic drugs[edit]
The serotonergic psychedelic drugs psilocin/psilocybin, DMT, mescaline, psychedelic mushroom and LSD are agonists, primarily at 5HT2A/2C receptors.[87][88][89] The empathogen-entactogen MDMA releases serotonin from synaptic vesicles of neurons.[90]
Antidepressants[edit]
Drugs that alter serotonin levels are used in treating depression, generalized anxiety disorder, and social phobia. Monoamine oxidase inhibitors (MAOIs) prevent the breakdown of monoamine neurotransmitters (including serotonin), and therefore increase concentrations of the neurotransmitter in the brain. MAOI therapy is associated with many adverse drug reactions, and patients are at risk of hypertensive emergency triggered by foods with high tyramine content, and certain drugs. Some drugs inhibit the re-uptake of serotonin, making it stay in the synaptic cleft longer. The tricyclic antidepressants (TCAs) inhibit the reuptake of both serotonin and norepinephrine. The newer selective serotonin reuptake inhibitors (SSRIs) have fewer side-effects and fewer interactions with other drugs.[91]
Certain SSRI medications have been shown to lower serotonin levels below the baseline after chronic use, despite initial increases.[92] The 5-HTTLPR gene codes for the number of serotonin transporters in the brain, with more serotonin transporters causing decreased duration and magnitude of serotonergic signaling.[93] The 5-HTTLPR polymorphism (l/l) causing more serotonin transporters to be formed is also found to be more resilient against depression and anxiety.[94][95]
Serotonin syndrome[edit]
Extremely high levels of serotonin can cause a condition known as serotonin syndrome, with toxic and potentially fatal effects. In practice, such toxic levels are essentially impossible to reach through an overdose of a single antidepressant drug, but require a combination of serotonergic agents, such as an SSRI with a MAOI, which may occur in therapeutic doses.[96][97] The intensity of the symptoms of serotonin syndrome vary over a wide spectrum, and the milder forms are seen even at nontoxic levels.[98] It is estimated that 14% of patients experiencing serotonin syndrome overdose on SSRIs; meanwhile the fatality rate is between 2% to 12%.[96][99][100]
Antiemetics[edit]
Some 5-HT3 antagonists, such as ondansetron, granisetron, and tropisetron, are important antiemetic agents. They are particularly important in treating the nausea and vomiting that occur during anticancer chemotherapy using cytotoxic drugs. Another application is in the treatment of postoperative nausea and vomiting.
Other[edit]
Some serotonergic agonist drugs cause fibrosis anywhere in the body, particularly the syndrome of retroperitoneal fibrosis, as well as cardiac valve fibrosis.[101]
In the past, three groups of serotonergic drugs have been epidemiologically linked with these syndromes. These are the serotonergic vasoconstrictive antimigraine drugs (ergotamine and methysergide),[101] the serotonergic appetite suppressant drugs (fenfluramine, chlorphentermine, and aminorex), and certain anti-Parkinsonian dopaminergic agonists, which also stimulate serotonergic 5-HT2B receptors. These include pergolide and cabergoline, but not the more dopamine-specific lisuride.[102]
As with fenfluramine, some of these drugs have been withdrawn from the market after groups taking them showed a statistical increase of one or more of the side effects described. An example is pergolide. The drug was declining in use since it was reported in 2003 to be associated with cardiac fibrosis.[103]
Two independent studies published in The New England Journal of Medicine in January 2007 implicated pergolide, along with cabergoline, in causing valvular heart disease.[104][105] As a result of this, the FDA removed pergolide from the United States market in March 2007.[106] (Since cabergoline is not approved in the United States for Parkinson’s Disease, but for hyperprolactinemia, the drug remains on the market. Treatment for hyperprolactinemia requires lower doses than that for Parkinson’s Disease, diminishing the risk of valvular heart disease).[107]
Methyl-tryptamines and hallucinogens[edit]
For details on tryptamine neurotransmitters in humans, see Trace amine.
Several plants contain serotonin together with a family of related tryptamines that are methylated at the amino (NH2) and (OH) groups, are N-oxides, or miss the OH group. These compounds do reach the brain, although some portion of them are metabolized by monoamine oxidase enzymes (mainly MAO-A) in the liver. Examples are plants from the genus Anadenanthera that are used in the hallucinogenic yopo snuff. These compounds are widely present in the leaves of many plants, and may serve as deterrents for animal ingestion. Serotonin occurs in several mushrooms of the genus Panaeolus.[108]
Comparative biology and evolution[edit]
Unicellular organisms[edit]
Serotonin is used by a variety of single-cell organisms for various purposes. SSRIs have been found to be toxic to algae.[109] The gastrointestinal parasite Entamoeba histolytica secretes serotonin, causing a sustained secretory diarrhea in some people.[23][110] Patients infected with E. histolytica have been found to have highly elevated serum serotonin levels, which returned to normal following resolution of the infection.[111] E. histolytica also responds to the presence of serotonin by becoming more virulent.[112] This means serotonin secretion not only serves to increase the spread of enteamoebas by giving the host diarrhea but also serves to coordinate their behaviour according to their population density, a phenomenon known as quorum sensing. Outside the gut of a host, there is nothing that the entoamoebas provoke to release serotonin, hence the serotonin concentration is very low. Low serotonin signals to the entoamoebas they are outside a host and they become less virulent to conserve energy. When they enter a new host, they multiply in the gut, and become more virulent as the enterochromaffine cells get provoked by them and the serotonin concentration increases.
Edible plants and mushrooms[edit]
In drying seeds, serotonin production is a way to get rid of the buildup of poisonous ammonia. The ammonia is collected and placed in the indole part of L-tryptophan, which is then decarboxylated by tryptophan decarboxylase to give tryptamine, which is then hydroxylated by a cytochrome P450 monooxygenase, yielding serotonin.[113]
However, since serotonin is a major gastrointestinal tract modulator, it may be produced in the fruits of plants as a way of speeding the passage of seeds through the digestive tract, in the same way as many well-known seed and fruit associated laxatives. Serotonin is found in mushrooms, fruits, and vegetables. The highest values of 25–400 mg/kg have been found in nuts of the walnut (Juglans) and hickory (Carya) genera. Serotonin concentrations of 3–30 mg/kg have been found in plantains, pineapples, banana, kiwifruit, plums, and tomatoes. Moderate levels from 0.1–3 mg/kg have been found in a wide range of tested vegetables.[24][20]
Serotonin is one compound of the poison contained in stinging nettles (Urtica dioica), where it causes pain on injection in the same manner as its presence in insect venoms (see below).[22] It is also naturally found in Paramuricea clavata, or the Red Sea Fan.[114]
Serotonin and tryptophan have been found in chocolate with varying cocoa contents. The highest serotonin content (2.93 µg/g) was found in chocolate with 85% cocoa, and the highest tryptophan content (13.27–13.34 µg/g) was found in 70–85% cocoa. The intermediate in the synthesis from tryptophan to serotonin, 5-hydroxytryptophan, was not found.[115]
Root development in Arabidopsis thaliana is stimulated and modulated by serotonin — in various ways at various concentrations.[116]
Serotonin serves as a plant defense chemical against fungi. When infected with Fusarium crown rot (Fusarium pseudograminearum), wheat (Triticum aestivum) greatly increases its production of tryptophan to synthesize new serotonin.[117] The function of this is poorly understood[117] but wheat also produces serotonin when infected by Stagonospora nodorum — in that case to retard spore production.[118] The model cereal Brachypodium distachyon — used as a research substitute for wheat and other production cereals — also produces serotonin, coumaroyl-serotonin, and feruloyl-serotonin in response to F. graminearum. This produces a slight antimicrobial effect. B. distachyon produces more serotonin (and conjugates) in response to deoxynivalenol (DON)-producing F. graminearum than non-DON-producing.[119] Solanum lycopersicum produces many AA conjugates — including several of serotonin — in its leaves, stems, and roots in response to Ralstonia solanacearum infection.[120]
Invertebrates[edit]
Serotonin functions as a neurotransmitter in the nervous systems of most animals.
Nematodes[edit]
For example, in the roundworm Caenorhabditis elegans, which feeds on bacteria, serotonin is released as a signal in response to positive events, such as finding a new source of food or in male animals finding a female with which to mate.[121] When a well-fed worm feels bacteria on its cuticle, dopamine is released, which slows it down; if it is starved, serotonin also is released, which slows the animal down further. This mechanism increases the amount of time animals spend in the presence of food.[122] The released serotonin activates the muscles used for feeding, while octopamine suppresses them.[123][124] Serotonin diffuses to serotonin-sensitive neurons, which control the animal’s perception of nutrient availability.
Decapods[edit]
If lobsters are injected with serotonin, they behave like dominant individuals whereas octopamine causes subordinate behavior.[25] A crayfish that is frightened may flip its tail to flee, and the effect of serotonin on this behavior depends largely on the animal’s social status. Serotonin inhibits the fleeing reaction in subordinates, but enhances it in socially dominant or isolated individuals. The reason for this is social experience alters the proportion between serotonin receptors (5-HT receptors) that have opposing effects on the fight-or-flight response.[clarification needed] The effect of 5-HT1 receptors predominates in subordinate animals, while 5-HT2 receptors predominates in dominants.[125]
In venoms[edit]
Serotonin is a common component of invertebrate venoms, salivary glands, nervous tissues, and various other tissues, across molluscs, insects, crustaceans, scorpions, various kinds of worms, and jellyfish.[22] Adult Rhodnius prolixus — hematophagous on vertebrates — secrete lipocalins into the wound during feeding. In 2003 these lipocalins were demonstrated to sequester serotonin to prevent vasoconstriction (and possibly coagulation) in the host.[126]
Insects[edit]
Serotonin is evolutionarily conserved and appears across the animal kingdom. It is seen in insect processes in roles similar to in the human central nervous system, such as memory, appetite, sleep, and behavior.[127][19] Some circuits in mushroom bodies are serotonergic.[128] (See specific Drosophila example below, §Dipterans.)
Acrididae[edit]
Locust swarming is initiated but not maintained by serotonin,[129] with release being triggered by tactile contact between individuals.[130] This transforms social preference from aversion to a gregarious state that enables coherent groups.[131][130][129] Learning in flies and honeybees is affected by the presence of serotonin.[132][133]
Role in insecticides[edit]
Insect 5-HT receptors have similar sequences to the vertebrate versions, but pharmacological differences have been seen. Invertebrate drug response has been far less characterized than mammalian pharmacology and the potential for species selective insecticides has been discussed.[134]
Hymenopterans[edit]
Wasps and hornets have serotonin in their venom,[135] which causes pain and inflammation[21][22] as do scorpions.[136][22] Pheidole dentata takes on more and more tasks in the colony as it gets older, which requires it to respond to more and more olfactory cues in the course of performing them. This olfactory response broadening was demonstrated to go along with increased serotonin and dopamine, but not octopamine in 2006.[137]
Dipterans[edit]
If flies are fed serotonin, they are more aggressive; flies depleted of serotonin still exhibit aggression, but they do so much less frequently.[138] In their crops it plays a vital role in digestive motility produced by contraction. Serotonin that acts on the crop is exogenous to the crop itself and 2012 research suggested that it probably originated in the serotonin neural plexus in the thoracic-abdominal synganglion.[139] In 2011 a Drosophila serotonergic mushroom body was found to work in concert with Amnesiac to form memories.[128] In 2007 serotonin was found to promote aggression in Diptera, which was counteracted by neuropeptide F — a surprising find given that they both promote courtship, which is usually similar to aggression in most respects.[128]
Vertebrates[edit]
Serotonin, also referred to as 5-hydroxytryptamine (5-HT), is a neurotransmitter most known for its involvement in mood disorders in humans. It is also a widely present neuromodulator among vertebrates and invertebrates.[140] Serotonin has been found having associations with many physiological systems such as cardiovascular, thermoregulation, and behavioral functions, including: circadian rhythm, appetite, aggressive and sexual behavior, sensorimotor reactivity and learning, and pain sensitivity.[141] Serotonin’s function in neurological systems along with specific behaviors among vertebrates found to be strongly associated with serotonin will be further discussed. Two relevant case studies are also mentioned regarding serotonin development involving teleost fish and mice.
In mammals, 5-HT is highly concentrated in the substantia nigra, ventral tegmental area and raphe nuclei. Lesser concentrated areas include other brain regions and the spinal cord.[140] 5-HT neurons are also shown to be highly branched, indicating that they are structurally prominent for influencing multiple areas of the CNS at the same time, although this trend is exclusive solely to mammals.[141]
5-HT System in Vertebrates[edit]
Vertebrates are multicellular organisms in the phylum Chordata that possess a backbone and a nervous system. This includes mammals, fish, reptiles, birds, etc. In humans, the nervous system is composed of the central and peripheral nervous system, with little known about the specific mechanisms of neurotransmitters in most other vertebrates. However, it is known that while serotonin is involved in stress and behavioral responses, it is also important in cognitive functions.[140] Brain organization in most vertebrates includes 5-HT cells in the hindbrain.[140] In addition to this, 5-HT is often found in other sections of the brain in non-placental vertebrates, including the basal forebrain and pretectum.[142] Since location of serotonin receptors contribute to behavioral responses, this suggests serotonin is part of specific pathways in non-placental vertebrates that are not present in amniotic organisms.[143] Teleost fish and mice are organisms most often used to study the connection between serotonin and vertebrate behavior. Both organisms show similarities in the effect of serotonin on behavior, but differ in the mechanism in which the responses occur.
Dogs / Canine species[edit]
There are few studies of serotonin in dogs. One study reported serotonin values were higher at dawn than at dusk.[144] In another study, serum 5-HT levels did not seem to be associated with dogs’ behavioural response to a stressful situation.[145] Urinary serotonin/creatinine ratio in bitches tended to be higher 4 weeks after surgery. In addition, serotonin was positively correlated with both cortisol and progesterone but not with testosterone after ovariohysterectomy.[146]
Teleost Fish[edit]
Like non-placental vertebrates, teleost fish also possess 5-HT cells in other sections of the brain, including the basal forebrain.[142] Danio rerio (zebra fish) are a species of teleost fish often used for studying serotonin within the brain. Despite much being unknown about serotonergic systems in vertebrates, the importance in moderating stress and social interaction is known.[147] It is hypothesized that AVT and CRF cooperate with serotonin in the hypothalamic-pituitary-interrenal axis.[142] These neuropeptides influence the plasticity of the teleost, affecting its ability to change and respond to its environment. Subordinate fish in social settings show a drastic increase in 5-HT concentrations.[147] High levels of 5-HT long term influence the inhibition of aggression in subordinate fish.[147]
Mice[edit]
Researchers at the Department of Pharmacology and Medical Chemistry used serotonergic drugs on male mice to study the effects of selected drugs on their behavior.[148] Mice in isolation exhibit increased levels of agonistic behavior towards one another. Results found that serotonergic drugs reduce aggression in isolated mice while simultaneously increasing social interaction.[148] Each of the treatments use a different mechanism for targeting aggression, but ultimately all have the same outcome. While the study shows that serotonergic drugs successfully target serotonin receptors, it does not show specifics of the mechanisms that affect behavior, as all types of drugs tended to reduce aggression in isolated male mice.[148] Aggressive mice kept out of isolation may respond differently to changes in serotonin reuptake.
Behavior[edit]
Like in humans, serotonin is extremely involved in regulating behavior in most other vertebrates. This includes not only response and social behaviors, but also influencing mood. Defects in serotonin pathways can lead to intense variations in mood, as well as symptoms of mood disorders, which can be present in more than just humans.
[edit]
One of the most researched aspects of social interaction in which serotonin is involved is aggression. Aggression is regulated by the 5-HT system, as serotonin levels can both induce or inhibit aggressive behaviors, as seen in mice (see section on Mice) and crabs.[148] While this is widely accepted, it is unknown if serotonin interacts directly or indirectly with parts of the brain influencing aggression and other behaviors.[140] Studies of serotonin levels show that they drastically increase and decrease during social interactions, and they generally correlate with inhibiting or inciting aggressive behavior.[149] The exact mechanism of serotonin influencing social behaviors is unknown, as pathways in the 5-HT system in various vertebrates can differ greatly.[140]
Response to Stimuli[edit]
Serotonin is important in environmental response pathways, along with other neurotransmitters.[150] Specifically, it has been found to be involved in auditory processing in social settings, as primary sensory systems are connected to social interactions.[151] Serotonin is found in the IC structure of the midbrain, which processes specie specific and non-specific social interactions and vocalizations.[151] It also receives acoustic projections that convey signals to auditory processing regions.[151] Research has proposed that serotonin shapes the auditory information being received by the IC and therefore is influential in the responses to auditory stimuli.[151] This can influence how an organism responds to the sounds of predatory or other impactful species in their environment, as serotonin uptake can influence aggression and/or social interaction.
Mood[edit]
We can describe mood not as specific to an emotional status, but as associated with a relatively long-lasting emotional state. Serotonin’s association with mood is most known for various forms of depression and bipolar disorders in humans.[141] Disorders caused by serotonergic activity potentially contribute to the many symptoms of major depression, such as overall mood, activity, suicidal thoughts and sexual and cognitive dysfunction. Selective serotonin reuptake inhibitors (SSRI’s) are a class of drugs demonstrated to be an effective treatment in major depressive disorder and are the most prescribed class of antidepressants. SSRI’s function is to block the reuptake of serotonin, making more serotonin available to absorb by the receiving neuron. Animals have been studied for decades in order to understand depressive behavior among species. One of the most familiar studies, the forced swimming test (FST), was performed to measure potential antidepressant activity.[141] Rats were placed in an inescapable container of water, at which point time spent immobile and number of active behaviors (such as splashing or climbing) were compared before and after a panel of anti-depressant drugs were administered. Antidepressants that selectively inhibit NE reuptake were shown to reduce immobility and selectively increase climbing without affecting swimming. However, results of the SSRI’s also show reduced immobility but increased swimming without affecting climbing. This study demonstrated the importance of behavioral tests for antidepressants, as they can detect drugs with an effect on core behavior along with behavioral components of species.[141]
Growth and reproduction[edit]
In the nematode C. elegans, artificial depletion of serotonin or the increase of octopamine cues behavior typical of a low-food environment: C. elegans becomes more active, and mating and egg-laying are suppressed, while the opposite occurs if serotonin is increased or octopamine is decreased in this animal.[27] Serotonin is necessary for normal nematode male mating behavior,[152] and the inclination to leave food to search for a mate.[153] The serotonergic signaling used to adapt the worm’s behaviour to fast changes in the environment affects insulin-like signaling and the TGF beta signaling pathway,[154] which control long-term adaption.
In the fruit fly insulin both regulates blood sugar as well as acting as a growth factor. Thus, in the fruit fly, serotonergic neurons regulate the adult body size by affecting insulin secretion.[155][156] Serotonin has also been identified as the trigger for swarm behavior in locusts.[131] In humans, though insulin regulates blood sugar and IGF regulates growth, serotonin controls the release of both hormones, modulating insulin release from the beta cells in the pancreas through serotonylation of GTPase signaling proteins.[31] Exposure to SSRIs during pregnancy reduces fetal growth.[157]
Genetically altered C. elegans worms that lack serotonin have an increased reproductive lifespan, may become obese, and sometimes present with arrested development at a dormant larval state.[158][159]
Aging and age-related phenotypes[edit]
Serotonin is known to regulate aging, learning and memory. The first evidence comes from the study of longevity in C. elegans.[154] During early phase of aging[vague], the level of serotonin increases, which alters locomotory behaviors and associative memory.[160] The effect is restored by mutations and drugs (including mianserin and methiothepin) that inhibit serotonin receptors. The observation does not contradict with the notion that the serotonin level goes down in mammals and humans, which is typically seen in late but not early[vague] phase of aging.
Biochemical mechanisms[edit]
Biosynthesis[edit]
The pathway for the synthesis of serotonin from tryptophan.
In animals including humans, serotonin is synthesized from the amino acid L-tryptophan by a short metabolic pathway consisting of two enzymes, tryptophan hydroxylase (TPH) and aromatic amino acid decarboxylase (DDC), and the coenzyme pyridoxal phosphate. The TPH-mediated reaction is the rate-limiting step in the pathway.
TPH has been shown to exist in two forms: TPH1, found in several tissues, and TPH2, which is a neuron-specific isoform.[161]
Serotonin can be synthesized from tryptophan in the lab using Aspergillus niger and Psilocybe coprophila as catalysts. The first phase to 5-hydroxytryptophan would require letting tryptophan sit in ethanol and water for 7 days, then mixing in enough HCl (or other acid) to bring the pH to 3, and then adding NaOH to make a pH of 13 for 1 hour. Asperigillus niger would be the catalyst for this first phase. The second phase to synthesizing tryptophan itself from the 5-hydroxytryptophan intermediate would require adding ethanol and water, and letting sit for 30 days this time. The next two steps would be the same as the first phase: adding HCl to make the pH = 3, and then adding NaOH to make the pH very basic at 13 for 1 hour. This phase uses the Psilocybe coprophila as the catalyst for the reaction.[162]
Serotonin taken orally does not pass into the serotonergic pathways of the central nervous system, because it does not cross the blood–brain barrier.[9] However, tryptophan and its metabolite 5-hydroxytryptophan (5-HTP), from which serotonin is synthesized, do cross the blood–brain barrier. These agents are available as dietary supplements and in various foods, and may be effective serotonergic agents.
One product of serotonin breakdown is 5-hydroxyindoleacetic acid (5-HIAA), which is excreted in the urine. Serotonin and 5-HIAA are sometimes produced in excess amounts by certain tumors or cancers, and levels of these substances may be measured in the urine to test for these tumors.
Analytical chemistry[edit]
Indium tin oxide is recommended for the electrode material in electrochemical investigation of concentrations produced, detected, or consumed by microbes.[163] A mass spectrometry technique was developed in 1994 to measure the molecular weight of both natural and synthetic serotonins.[164]
History and etymology[edit]
It had been known to physiologists for over a century that a vasoconstrictor material appears in serum when blood was allowed to clot.[165] In 1935, Italian Vittorio Erspamer showed an extract from enterochromaffin cells made intestines contract. Some believed it contained adrenaline, but two years later, Erspamer was able to show it was a previously unknown amine, which he named «enteramine».[166][167] In 1948, Maurice M. Rapport, Arda Green, and Irvine Page of the Cleveland Clinic discovered a vasoconstrictor substance in blood serum, and since it was a serum agent affecting vascular tone, they named it serotonin.[168]
In 1952, enteramine was shown to be the same substance as serotonin, and as the broad range of physiological roles was elucidated, the abbreviation 5-HT of the proper chemical name 5-hydroxytryptamine became the preferred name in the pharmacological field.[169] Synonyms of serotonin include: 5-hydroxytriptamine, thrombotin, enteramin, substance DS, and 3-(β-Aminoethyl)-5-hydroxyindole.[170] In 1953, Betty Twarog and Page discovered serotonin in the central nervous system.[171] Page regarded Erspamer’s work on Octopus vulgaris, Discoglossus pictus, Hexaplex trunculus, Bolinus brandaris, Sepia, Mytilus, and Ostrea as valid and fundamental to understanding this newly identified substance, but regarded his earlier results in various models — especially those from rat blood — to be too confounded by the presence of other MAs, including some other vasoactives.[172]
See also[edit]
- HIOC
Notes[edit]
- ^ References for the functions of these receptors are available on the wikipedia pages for the specific receptor in question
References[edit]
- ^ Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (©1994–2011 ACD/Labs)
- ^ Mazák K, Dóczy V, Kökösi J, Noszál B (April 2009). «Proton speciation and microspeciation of serotonin and 5-hydroxytryptophan». Chemistry & Biodiversity. 6 (4): 578–590. doi:10.1002/cbdv.200800087. PMID 19353542. S2CID 20543931.
- ^ Pietra S (1958). «[Indolic derivatives. II. A new way to synthesize serotonin]». Il Farmaco; Edizione Scientifica (in Italian). 13 (1): 75–79. PMID 13524273.
- ^ Erspamer V (1952). «Ricerche preliminari sulle indolalchilamine e sulle fenilalchilamine degli estratti di pelle di Anfibio». Ricerca Scientifica. 22: 694–702.
- ^ Tammisto T (1967). «Increased toxicity of 5-hydroxytryptamine by ethanol in rats and mice». Annales Medicinae Experimentalis et Biologiae Fenniae. 46 (3): 382–384. PMID 5734241.
- ^ Jones D (2003) [1917]. Roach P, Hartmann J, Setter J (eds.). English Pronouncing Dictionary. Cambridge: Cambridge University Press. ISBN 978-3-12-539683-8.
- ^ «Serotonin». Dictionary.com Unabridged (Online). n.d.
- ^ «Serotonin». Merriam-Webster Dictionary.
- ^ a b Young SN (November 2007). «How to increase serotonin in the human brain without drugs». Journal of Psychiatry & Neuroscience. 32 (6): 394–399. PMC 2077351. PMID 18043762.
- ^ «Microbes Help Produce Serotonin in Gut». California Institute of Technology. 9 April 2015. Retrieved 3 June 2022.
- ^ González-Flores D, Velardo B, Garrido M, et al. (2011). «Ingestion of Japanese plums (Prunus salicina Lindl. cv. Crimson Globe) increases the urinary 6-sulfatoxymelatonin and total antioxidant capacity levels in young, middle-aged and elderly humans: Nutritional and functional characterization of their content». Journal of Food and Nutrition Research. 50 (4): 229–236.
- ^ Schlienger RG, Meier CR (2003). «Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction?». American Journal of Cardiovascular Drugs: Drugs, Devices, and Other Interventions. 3 (3): 149–162. doi:10.2165/00129784-200303030-00001. PMID 14727927. S2CID 23986530.
- ^ King MW. «Serotonin». The Medical Biochemistry Page. Indiana University School of Medicine. Retrieved 1 December 2009.
- ^ Berger M, Gray JA, Roth BL (2009). «The expanded biology of serotonin». Annual Review of Medicine. 60: 355–366. doi:10.1146/annurev.med.60.042307.110802. PMC 5864293. PMID 19630576.
- ^ Kling A (2013). 5-HT2A: a serotonin receptor with a possible role in joint diseases (PDF) (Thesis). Umeå Universitet. ISBN 978-91-7459-549-9.
- ^ Yano JM, Yu K, Donaldson GP, et al. (April 2015). «Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis». Cell. 161 (2): 264–276. doi:10.1016/j.cell.2015.02.047. PMC 4393509. PMID 25860609.
- ^ Vanhoutte PM (February 1987). «Serotonin and the vascular wall». International Journal of Cardiology. 14 (2): 189–203. doi:10.1016/0167-5273(87)90008-8. PMID 3818135.
- ^ Prah A, Purg M, Stare J, et al. (September 2020). «How Monoamine Oxidase A Decomposes Serotonin: An Empirical Valence Bond Simulation of the Reactive Step». The Journal of Physical Chemistry B. 124 (38): 8259–8265. doi:10.1021/acs.jpcb.0c06502. PMC 7520887. PMID 32845149.
- ^ a b Huser A, Rohwedder A, Apostolopoulou AA, Widmann A, Pfitzenmaier JE, Maiolo EM, et al. (2012). Zars T (ed.). «The serotonergic central nervous system of the Drosophila larva: anatomy and behavioral function». PLOS ONE. 7 (10): e47518. Bibcode:2012PLoSO…747518H. doi:10.1371/journal.pone.0047518. PMC 3474743. PMID 23082175.
- ^ a b c Ramakrishna A, Giridhar P, Ravishankar GA (June 2011). «Phytoserotonin: a review». Plant Signaling & Behavior. 6 (6): 800–809. doi:10.4161/psb.6.6.15242. PMC 3218476. PMID 21617371.
- ^ a b Chen J, Lariviere WR (October 2010). «The nociceptive and anti-nociceptive effects of bee venom injection and therapy: a double-edged sword». Progress in Neurobiology. 92 (2): 151–183. doi:10.1016/j.pneurobio.2010.06.006. PMC 2946189. PMID 20558236.
- ^ a b c d e Erspamer V (1966). «Occurrence of indolealkylamines in nature». 5-Hydroxytryptamine and Related Indolealkylamines. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 132–181. doi:10.1007/978-3-642-85467-5_4. ISBN 978-3-642-85469-9.
- ^ a b McGowan K, Kane A, Asarkof N, et al. (August 1983). «Entamoeba histolytica causes intestinal secretion: role of serotonin». Science. 221 (4612): 762–764. Bibcode:1983Sci…221..762M. doi:10.1126/science.6308760. PMID 6308760.
- ^ a b Feldman JM, Lee EM (October 1985). «Serotonin content of foods: effect on urinary excretion of 5-hydroxyindoleacetic acid». The American Journal of Clinical Nutrition. 42 (4): 639–643. doi:10.1093/ajcn/42.4.639. PMID 2413754.
- ^ a b Kravitz EA (September 1988). «Hormonal control of behavior: amines and the biasing of behavioral output in lobsters». Science. 241 (4874): 1775–1781. Bibcode:1988Sci…241.1775K. doi:10.1126/science.2902685. PMID 2902685.
- ^ a b Zifa E, Fillion G (September 1992). «5-Hydroxytryptamine receptors». Pharmacological Reviews. 44 (3): 401–458. PMID 1359584.
- ^ a b Srinivasan S, Sadegh L, Elle IC, et al. (June 2008). «Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms». Cell Metabolism. 7 (6): 533–544. doi:10.1016/j.cmet.2008.04.012. PMC 2495008. PMID 18522834.
- ^ Ramakrishna A, Ravishankar GA (November 2011). «Influence of abiotic stress signals on secondary metabolites in plants». Plant Signaling & Behavior. Informa. 6 (11): 1720–1731. doi:10.4161/psb.6.11.17613. PMC 3329344. PMID 22041989.
- ^ Hannon J, Hoyer D (December 2008). «Molecular biology of 5-HT receptors». Behavioural Brain Research. 195 (1): 198–213. doi:10.1016/j.bbr.2008.03.020. PMID 18571247. S2CID 46043982.
- ^ a b c Zhou M, Engel K, Wang J (January 2007). «Evidence for significant contribution of a newly identified monoamine transporter (PMAT) to serotonin uptake in the human brain». Biochemical Pharmacology. 73 (1): 147–154. doi:10.1016/j.bcp.2006.09.008. PMC 1828907. PMID 17046718.
- ^ a b c Paulmann N, Grohmann M, Voigt JP, et al. (October 2009). O’Rahilly S (ed.). «Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation». PLOS Biology. 7 (10): e1000229. doi:10.1371/journal.pbio.1000229. PMC 2760755. PMID 19859528.
- ^ Walther DJ, Peter JU, Winter S, et al. (December 2003). «Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release». Cell. 115 (7): 851–862. doi:10.1016/S0092-8674(03)01014-6. PMID 14697203. S2CID 16847296.
- ^ Watts SW, Priestley JR, Thompson JM (May 2009). «Serotonylation of vascular proteins important to contraction». PLOS ONE. 4 (5): e5682. Bibcode:2009PLoSO…4.5682W. doi:10.1371/journal.pone.0005682. PMC 2682564. PMID 19479059.
- ^ Roth BL, Driscol J (12 January 2011). «PDSP Ki Database». Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 8 November 2013. Retrieved 17 December 2013.
- ^ Bortolozzi A, Díaz-Mataix L, Scorza MC, et al. (December 2005). «The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity». Journal of Neurochemistry. 95 (6): 1597–1607. doi:10.1111/j.1471-4159.2005.03485.x. hdl:10261/33026. PMID 16277612. S2CID 18350703.
- ^ Moro C, Edwards L, Chess-Williams R (November 2016). «2Areceptor enhancement of contractile activity of the porcine urothelium and lamina propria». International Journal of Urology. 23 (11): 946–951. doi:10.1111/iju.13172. PMID 27531585.
- ^ «Von Economo neuron – NeuronBank». neuronbank.org.[unreliable medical source?]
- ^ Millan MJ, Gobert A, Lejeune F, et al. (September 2003). «The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways». The Journal of Pharmacology and Experimental Therapeutics. 306 (3): 954–964. doi:10.1124/jpet.103.051797. PMID 12750432. S2CID 18753440.
- ^ Gonzalez R, Chávez-Pascacio K, Meneses A (September 2013). «Role of 5-HT5A receptors in the consolidation of memory». Behavioural Brain Research. 252: 246–251. doi:10.1016/j.bbr.2013.05.051. PMID 23735322. S2CID 140204585.
- ^ Nautiyal KM, Hen R (2017). «Serotonin receptors in depression: from A to B». F1000Research. 6: 123. doi:10.12688/f1000research.9736.1. PMC 5302148. PMID 28232871.
- ^ Frazer A, Hensler JG (1999). «Understanding the neuroanatomical organization of serotonergic cells in the brain provides insight into the functions of this neurotransmitter». In Siegel GJ, Agranoff, Bernard W, Fisher SK, Albers RW, Uhler MD (eds.). Basic Neurochemistry (Sixth ed.). Lippincott Williams & Wilkins. ISBN 978-0-397-51820-3.
In 1964, Dahlstrom and Fuxe (discussed in [2]), using the Falck-Hillarp technique of histofluorescence, observed that the majority of serotonergic soma are found in cell body groups, which previously had been designated as the Raphe nuclei.
- ^ Binder MD, Hirokawa N (2009). encyclopedia of neuroscience. Berlin: Springer. p. 705. ISBN 978-3-540-23735-8.
- ^ The raphe nuclei group of neurons are located along the brain stem from the labels ‘Mid Brain’ to ‘Oblongata’, centered on the pons. (See relevant image.)
- ^ Müller CP, Jacobs BL, eds. (2009). Handbook of the behavioral neurobiology of serotonin (1st ed.). London: Academic. pp. 51–59. ISBN 978-0123746344.
- ^ Passos AD, Herculano AM, Oliveira KR, de Lima SM, Rocha FA, Freitas HR, et al. (October 2019). «Regulation of the Serotonergic System by Kainate in the Avian Retina». Cellular and Molecular Neurobiology. 39 (7): 1039–1049. doi:10.1007/s10571-019-00701-8. PMID 31197744. S2CID 189763144.
- ^ Sommer C (2009). «Serotonin in Pain and Pain Control». In Müller CP, Jacobs BL (eds.). Handbook of the behavioral neurobiology of serotonin (1st ed.). London: Academic. pp. 457–460. ISBN 978-0123746344.
- ^ Hensler JG (2009). «Serotonin in Mode and Emotions». In Müller CP, Jacobs BL (eds.). Handbook of the behavioral neurobiology of serotonin (1st ed.). London: Academic. pp. 367–399. ISBN 978-0123746344.
- ^ Andrews PW, Bharwani A, Lee KR, et al. (April 2015). «Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response». Neuroscience and Biobehavioral Reviews. 51: 164–188. doi:10.1016/j.neubiorev.2015.01.018. PMID 25625874. S2CID 23980182.
- ^ Stahl SM, Mignon L, Meyer JM (March 2009). «Which comes first: atypical antipsychotic treatment or cardiometabolic risk?». Acta Psychiatrica Scandinavica. 119 (3): 171–179. doi:10.1111/j.1600-0447.2008.01334.x. PMID 19178394. S2CID 24035040.
- ^ Buckland PR, Hoogendoorn B, Guy CA, et al. (March 2005). «Low gene expression conferred by association of an allele of the 5-HT2C receptor gene with antipsychotic-induced weight gain». The American Journal of Psychiatry. 162 (3): 613–615. doi:10.1176/appi.ajp.162.3.613. PMID 15741483.
- ^ Holmes MC, French KL, Seckl JR (June 1997). «Dysregulation of diurnal rhythms of serotonin 5-HT2C and corticosteroid receptor gene expression in the hippocampus with food restriction and glucocorticoids». The Journal of Neuroscience. 17 (11): 4056–4065. doi:10.1523/JNEUROSCI.17-11-04056.1997. PMC 6573558. PMID 9151722.
- ^ Leibowitz SF (1990). «The role of serotonin in eating disorders». Drugs. 39 (Suppl 3): 33–48. doi:10.2165/00003495-199000393-00005. PMID 2197074. S2CID 8612545.
- ^ McGuire, Michael (2013) «Believing, the neuroscience of fantasies, fears, and confictions» (Prometius Books)
- ^ Caspi N, Modai I, Barak P, et al. (March 2001). «Pindolol augmentation in aggressive schizophrenic patients: a double-blind crossover randomized study». International Clinical Psychopharmacology. 16 (2): 111–115. doi:10.1097/00004850-200103000-00006. PMID 11236069. S2CID 24822810.
- ^ Ito Z, Aizawa I, Takeuchi M, Tabe M, Nakamura T (December 1975). «[Proceedings: Study of gastrointestinal motility using an extraluminal force transducer. 6. Observation of gastric and duodenal motility using synthetic motilin]». Nihon Heikatsukin Gakkai Zasshi. 11 (4): 244–246. PMID 1232434.
- ^ Lesch KP, Bengel D, Heils A, et al. (November 1996). «Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region». Science. 274 (5292): 1527–1531. Bibcode:1996Sci…274.1527L. doi:10.1126/science.274.5292.1527. PMID 8929413. S2CID 35503987.
- ^ Chilmonczyk Z, Bojarski AJ, Pilc A, Sylte I (August 2015). «Functional Selectivity and Antidepressant Activity of Serotonin 1A Receptor Ligands». International Journal of Molecular Sciences. 16 (8): 18474–18506. doi:10.3390/ijms160818474. PMC 4581256. PMID 26262615.
- ^ Blier P, El Mansari M (2013). «Serotonin and beyond: therapeutics for major depression». Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 368 (1615): 20120536. doi:10.1098/rstb.2012.0536. PMC 3638389. PMID 23440470.
- ^ a b c d Eissa N, Al-Houqani M, Sadeq A, Ojha SK, Sasse A, Sadek B (16 May 2018). «Current Enlightenment About Etiology and Pharmacological Treatment of Autism Spectrum Disorder». Frontiers in Neuroscience. 12: 304. doi:10.3389/fnins.2018.00304. PMC 5964170. PMID 29867317.
- ^ Beattie DT, Smith JA (May 2008). «Serotonin pharmacology in the gastrointestinal tract: a review». Naunyn-Schmiedeberg’s Archives of Pharmacology. 377 (3): 181–203. doi:10.1007/s00210-008-0276-9. PMID 18398601. S2CID 32820765.
- ^ Rang HP (2003). Pharmacology. Edinburgh: Churchill Livingstone. p. 187. ISBN 978-0-443-07145-4.
- ^ de Wit R, Aapro M, Blower PR (September 2005). «Is there a pharmacological basis for differences in 5-HT3-receptor antagonist efficacy in refractory patients?». Cancer Chemotherapy and Pharmacology. 56 (3): 231–238. doi:10.1007/s00280-005-1033-0. PMID 15838653. S2CID 27576150.
- ^ Frost M, Andersen TE, Yadav V, et al. (March 2010). «Patients with high-bone-mass phenotype owing to Lrp5-T253I mutation have low plasma levels of serotonin». Journal of Bone and Mineral Research. 25 (3): 673–675. doi:10.1002/jbmr.44. PMID 20200960. S2CID 24280062.
- ^ Rosen CJ (February 2009). «Breaking into bone biology: serotonin’s secrets». Nature Medicine. 15 (2): 145–146. doi:10.1038/nm0209-145. PMID 19197289. S2CID 5489589.
- ^ Mödder UI, Achenbach SJ, Amin S, et al. (February 2010). «Relation of serum serotonin levels to bone density and structural parameters in women». Journal of Bone and Mineral Research. 25 (2): 415–422. doi:10.1359/jbmr.090721. PMC 3153390. PMID 19594297.
- ^ Frost M, Andersen T, Gossiel F, et al. (August 2011). «Levels of serotonin, sclerostin, bone turnover markers as well as bone density and microarchitecture in patients with high-bone-mass phenotype due to a mutation in Lrp5». Journal of Bone and Mineral Research. 26 (8): 1721–1728. doi:10.1002/jbmr.376. PMID 21351148.
- ^ Kode A, Mosialou I, Silva BC, et al. (October 2012). «FOXO1 orchestrates the bone-suppressing function of gut-derived serotonin». The Journal of Clinical Investigation. 122 (10): 3490–3503. doi:10.1172/JCI64906. PMC 3461930. PMID 22945629.
- ^ Yadav VK, Balaji S, Suresh PS, et al. (March 2010). «Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis». Nature Medicine. 16 (3): 308–312. doi:10.1038/nm.2098. PMC 2836724. PMID 20139991.
- ^ Sugisawa E, Takayama Y, Takemura N, et al. (July 2019). «RNA Sensing by Gut Piezo1 Is Essential for Systemic Serotonin Synthesis». Cell. 182 (3): 609–624. doi:10.1016/j.cell.2020.06.022. PMID 32640190.
- ^ Ozanne SE, Hales CN (January 2004). «Lifespan: catch-up growth and obesity in male mice». Nature. 427 (6973): 411–412. Bibcode:2004Natur.427..411O. doi:10.1038/427411b. PMID 14749819. S2CID 40256021.
- ^ Lewis DS, Bertrand HA, McMahan CA, et al. (October 1986). «Preweaning food intake influences the adiposity of young adult baboons». J. Clin. Invest. 78 (4): 899–905. doi:10.1172/JCI112678. PMC 423712. PMID 3760191.
- ^ Hahn P (July 1984). «Effect of litter size on plasma cholesterol and insulin and some liver and adipose tissue enzymes in adult rodents». J. Nutr. 114 (7): 1231–1234. doi:10.1093/jn/114.7.1231. PMID 6376732.
- ^ Popa D, Léna C, Alexandre C, Adrien J (April 2008). «Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior». The Journal of Neuroscience. 28 (14): 3546–3554. doi:10.1523/JNEUROSCI.4006-07.2008. PMC 6671102. PMID 18385313.
- ^ Maciag D, Simpson KL, Coppinger D, et al. (January 2006). «Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry». Neuropsychopharmacology. 31 (1): 47–57. doi:10.1038/sj.npp.1300823. PMC 3118509. PMID 16012532.
- ^ Maciag D, Williams L, Coppinger D, Paul IA (February 2006). «Neonatal citalopram exposure produces lasting changes in behavior which are reversed by adult imipramine treatment». European Journal of Pharmacology. 532 (3): 265–269. doi:10.1016/j.ejphar.2005.12.081. PMC 2921633. PMID 16483567.
- ^ Holden C (October 2004). «Neuroscience. Prozac treatment of newborn mice raises anxiety». Science. 306 (5697): 792. doi:10.1126/science.306.5697.792. PMID 15514122.
- ^ Ansorge MS, Zhou M, Lira A, et al. (October 2004). «Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice». Science. 306 (5697): 879–881. Bibcode:2004Sci…306..879A. doi:10.1126/science.1101678. PMID 15514160.
- ^ Lesurtel M, Graf R, Aleil B, et al. (April 2006). «Platelet-derived serotonin mediates liver regeneration». Science. 312 (5770): 104–107. Bibcode:2006Sci…312..104L. doi:10.1126/science.1123842. PMID 16601191. S2CID 43189753.
- ^ Matondo RB, Punt C, Homberg J, et al. (April 2009). «Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration». American Journal of Physiology. Gastrointestinal and Liver Physiology. 296 (4): G963–G968. doi:10.1152/ajpgi.90709.2008. PMID 19246633.
- ^ Collet C, Schiltz C, Geoffroy V, et al. (February 2008). «The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation». FASEB Journal. 22 (2): 418–427. doi:10.1096/fj.07-9209com. PMC 5409955. PMID 17846081.
- ^ Yadav VK, Ryu JH, Suda N, et al. (November 2008). «Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum». Cell. 135 (5): 825–837. doi:10.1016/j.cell.2008.09.059. PMC 2614332. PMID 19041748.
- «It Takes Guts To Build Bone, Scientists Discover». ScienceDaily (Press release). 1 December 2008.
- ^ McDuffie JE, Motley ED, Limbird LE, Maleque MA (March 2000). «5-hydroxytryptamine stimulates phosphorylation of p44/p42 mitogen-activated protein kinase activation in bovine aortic endothelial cell cultures». Journal of Cardiovascular Pharmacology. 35 (3): 398–402. doi:10.1097/00005344-200003000-00008. PMID 10710124.
- ^ Marieb EN (2009). Essentials of Human Anatomy & Physiology (Eighth ed.). San Francisco: Pearson/Benjamin Cummings. p. 336. ISBN 978-0-321-51342-7.
- ^ Chang W, Kanda H, Ikeda R, Ling J, DeBerry JJ, Gu JG (September 2016). «Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals». Proceedings of the National Academy of Sciences of the United States of America. 113 (37): E5491-500. Bibcode:2016PNAS..113E5491C. doi:10.1073/pnas.1610176113. PMC 5027443. PMID 27573850.
- ^ Noguchi M, Furukawa KT, Morimoto M (December 2020). «Pulmonary neuroendocrine cells: physiology, tissue homeostasis and disease». Disease Models & Mechanisms. 13 (12): dmm046920. doi:10.1242/dmm.046920. PMC 7774893. PMID 33355253.
- ^ Fuller RW (1980). «Pharmacology of central serotonin neurons». Annual Review of Pharmacology and Toxicology. 20: 111–127. doi:10.1146/annurev.pa.20.040180.000551. PMID 6992697.
- ^ Titeler M, Lyon RA, Glennon RA (1988). «Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens». Psychopharmacology. 94 (2): 213–216. doi:10.1007/BF00176847. PMID 3127847. S2CID 24179554.
- ^ Nichols DE (2000). «Role of serotonergic neurons and 5-HT receptors in the action of hallucinogens». In Baumgarten HG, Gothert M (eds.). Serotoninergic Neurons and 5-HT Receptors in the CNS. Santa Clara, CA: Springer-Verlag TELOS. ISBN 978-3-540-66715-5.
- ^ Kapur S, Seeman P (2002). «NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia». Molecular Psychiatry. 7 (8): 837–844. doi:10.1038/sj.mp.4001093. PMID 12232776.
- ^ Johnson MP, Hoffman AJ, Nichols DE (December 1986). «Effects of the enantiomers of MDA, MDMA and related analogues on [3H]serotonin and [3H]dopamine release from superfused rat brain slices». European Journal of Pharmacology. 132 (2–3): 269–276. doi:10.1016/0014-2999(86)90615-1. PMID 2880735.
- ^ Goodman LS, Brunton LL, Chabner B, Knollmann BC (2001). Goodman and Gilman’s pharmacological basis of therapeutics. New York: McGraw-Hill. pp. 459–461. ISBN 978-0-07-162442-8.
- ^ Benmansour S, Cecchi M, Morilak DA, et al. (December 1999). «Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level». The Journal of Neuroscience. 19 (23): 10494–10501. doi:10.1523/JNEUROSCI.19-23-10494.1999. PMC 6782424. PMID 10575045.
- ^ Beitchman JH, Baldassarra L, Mik H, et al. (June 2006). «Serotonin transporter polymorphisms and persistent, pervasive childhood aggression». The American Journal of Psychiatry. 163 (6): 1103–1105. doi:10.1176/appi.ajp.163.6.1103. PMID 16741214.
- ^ Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. (June 2005). «5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression». Nature Neuroscience. 8 (6): 828–834. doi:10.1038/nn1463. PMID 15880108. S2CID 1864631.
- ^ Schinka JA, Busch RM, Robichaux-Keene N (February 2004). «A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety». Molecular Psychiatry. 9 (2): 197–202. doi:10.1038/sj.mp.4001405. PMID 14966478.
- ^ a b New AM, Nelson S, Leung JG (1 October 2015). Alexander E, Susla GM (eds.). «Psychiatric Emergencies in the Intensive Care Unit». AACN Advanced Critical Care. 26 (4): 285–293, quiz 294–295. doi:10.4037/NCI.0000000000000104. PMID 26484986.
- ^ Isbister GK, Bowe SJ, Dawson A, Whyte IM (2004). «Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose». Journal of Toxicology. Clinical Toxicology. 42 (3): 277–285. doi:10.1081/CLT-120037428. PMID 15362595. S2CID 43121327.
- ^ Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM (September 2003). «The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity». QJM. 96 (9): 635–642. doi:10.1093/qjmed/hcg109. PMID 12925718.
- ^ Frank C (July 2008). «Recognition and treatment of serotonin syndrome». Canadian Family Physician. 54 (7): 988–992. PMC 2464814. PMID 18625822.
- ^ Boyer EW, Shannon M (March 2005). «The serotonin syndrome». The New England Journal of Medicine. 352 (11): 1112–1120. doi:10.1056/NEJMra041867. PMID 15784664.
- ^ a b Baskin SI (1991). Principles of cardiac toxicology. Boca Raton: CRC Press. ISBN 978-0-8493-8809-5. Retrieved 3 February 2010.
- ^ Jähnichen S, Horowski R, Pertz H. «Pergolide and Cabergoline But not Lisuride Exhibit Agonist Efficacy at Serotonin 5-HT2B Receptors» (PDF). Retrieved 3 February 2010.
- ^ Adverse Drug Reactions Advisory Committee, Australia (2004). «Cardiac valvulopathy with pergolide». Aust Adv Drug React Bull. 23 (4). Archived from the original on 27 June 2012.
- ^ Schade R, Andersohn F, Suissa S, et al. (January 2007). «Dopamine agonists and the risk of cardiac-valve regurgitation». The New England Journal of Medicine. 356 (1): 29–38. doi:10.1056/NEJMoa062222. PMID 17202453.
- ^ Zanettini R, Antonini A, Gatto G, et al. (January 2007). «Valvular heart disease and the use of dopamine agonists for Parkinson’s disease». The New England Journal of Medicine. 356 (1): 39–46. doi:10.1056/NEJMoa054830. PMID 17202454.
- ^ «Food and Drug Administration Public Health Advisory». Food and Drug Administration. 29 March 2007. Retrieved 7 February 2010.
- ^ «MedWatch – 2007 Safety Information Alerts. Permax (pergolide) and generic equivalents». United States Food and Drug Administration. 29 March 2007. Retrieved 30 March 2007.
- ^ Tyler VE (September 1958). «Occurrence of serotonin in a hallucinogenic mushroom». Science. 128 (3326): 718. Bibcode:1958Sci…128..718T. doi:10.1126/science.128.3326.718. PMID 13580242.
- ^ Johnson DJ, Sanderson H, Brain RA, et al. (May 2007). «Toxicity and hazard of selective serotonin reuptake inhibitor antidepressants fluoxetine, fluvoxamine, and sertraline to algae». Ecotoxicology and Environmental Safety. 67 (1): 128–139. doi:10.1016/j.ecoenv.2006.03.016. PMID 16753215.
- ^ McGowan K, Guerina V, Wicks J, Donowitz M (1985). «Secretory Hormones of Entamoeba histolytica». Secretory hormones of Entamoeba histolytica. Ciba Foundation Symposium. Novartis Foundation Symposia. Vol. 112. pp. 139–154. doi:10.1002/9780470720936.ch8. ISBN 978-0470720936. PMID 2861068.
- ^ Banu N, Zaidi KR, Mehdi G, Mansoor T (July 2005). «Neurohumoral alterations and their role in amoebiasis». Indian Journal of Clinical Biochemistry. 20 (2): 142–145. doi:10.1007/BF02867414. PMC 3453840. PMID 23105547.
- ^ Acharya DP, Sen MR, Sen PC (August 1989). «Effect of exogenous 5-hydroxytryptamine on pathogenicity of Entamoeba histolytica in experimental animals». Indian Journal of Experimental Biology. 27 (8): 718–720. PMID 2561282.
- ^ Schröder P, Abele C, Gohr P, Stuhlfauth-Roisch U, Grosse W (1999). «Latest on enzymology of serotonin biosynthesis in walnut seeds». Tryptophan, Serotonin, and Melatonin. Advances in Experimental Medicine and Biology. Vol. 467. pp. 637–644. doi:10.1007/978-1-4615-4709-9_81. ISBN 978-0-306-46204-7. PMID 10721112.
- ^ Pénez N, Culioli G, Pérez T, et al. (October 2011). «Antifouling properties of simple indole and purine alkaloids from the Mediterranean gorgonian Paramuricea clavata«. Journal of Natural Products. 74 (10): 2304–2308. doi:10.1021/np200537v. PMID 21939218.
- ^ Guillén-Casla V, Rosales-Conrado N, León-González ME, et al. (April 2012). «Determination of serotonin and its precursors in chocolate samples by capillary liquid chromatography with mass spectrometry detection». Journal of Chromatography A. 1232: 158–165. doi:10.1016/j.chroma.2011.11.037. PMID 22186492.
- ^ Pelagio-Flores R, Ortíz-Castro R, Méndez-Bravo A, Macías-Rodríguez L, López-Bucio J (March 2011). «Serotonin, a tryptophan-derived signal conserved in plants and animals, regulates root system architecture probably acting as a natural auxin inhibitor in Arabidopsis thaliana». Plant & Cell Physiology. Oxford University Press (OUP). 52 (3): 490–508. doi:10.1093/pcp/pcr006. PMID 21252298.
- ^ a b Powell JJ, Carere J, Fitzgerald TL, Stiller J, Covarelli L, Xu Q, et al. (March 2017). «The Fusarium crown rot pathogen Fusarium pseudograminearum triggers a suite of transcriptional and metabolic changes in bread wheat (Triticum aestivum L.)». Annals of Botany. Oxford University Press (OUP). 119 (5): 853–867. doi:10.1093/aob/mcw207. PMC 5604588. PMID 27941094. S2CID 3823345.
- ^ Du Fall LA, Solomon PS (October 2013). «The necrotrophic effector SnToxA induces the synthesis of a novel phytoalexin in wheat». The New Phytologist. Wiley. 200 (1): 185–200. doi:10.1111/nph.12356. PMID 23782173.
- ^ Pasquet JC, Chaouch S, Macadré C, Balzergue S, Huguet S, Martin-Magniette ML, et al. (July 2014). «Differential gene expression and metabolomic analyses of Brachypodium distachyon infected by deoxynivalenol producing and non-producing strains of Fusarium graminearum». BMC Genomics. BioMed Central. 15 (1): 629. doi:10.1186/1471-2164-15-629. PMC 4124148. PMID 25063396.
- ^ Zeiss DR, Piater LA, Dubery IA (2021). «Hydroxycinnamate Amides: Intriguing Conjugates of Plant Protective Metabolites». Trends in Plant Science. Cell Press. 26 (2): 184–195. doi:10.1016/j.tplants.2020.09.011. ISSN 1360-1385. PMID 33036915. S2CID 222256660.
- ^ Jonz MG, EkateriniMercier A, JoffrePotter JW (2001). «Effects Of 5-HT (Serotonin) On Reproductive Behaviour In Heterodera Schachtii (Nematoda)». Canadian Journal of Zoology. 79 (9): 1727. doi:10.1139/z01-135.
- ^ Sawin ER, Ranganathan R, Horvitz HR (June 2000). «C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway». Neuron. 26 (3): 619–631. doi:10.1016/S0896-6273(00)81199-X. PMID 10896158. S2CID 9247380.
- ^ Niacaris T, Avery L (January 2003). «Serotonin regulates repolarization of the C. elegans pharyngeal muscle». The Journal of Experimental Biology. 206 (Pt 2): 223–231. doi:10.1242/jeb.00101. PMC 4441752. PMID 12477893.
- ^ Rosso MN, Jones JT, Abad P (2009). «RNAi and functional genomics in plant parasitic nematodes». Annual Review of Phytopathology. Annual Reviews. 47 (1): 207–232. doi:10.1146/annurev.phyto.112408.132605. PMID 19400649. p. 218:
Octopamine and serotonin regulates the activity of the M3 neurons that direct contraction of the pharynx during C. elegans feeding… Soaking Meloidogyne J2 in dsRNA in the presence of … resorcinol plus serotonin resulted in uptake of solutions and silencing of genes expressed in the intestine and esophageal glands.
- ^ Yeh SR, Fricke RA, Edwards DH (January 1996). «The effect of social experience on serotonergic modulation of the escape circuit of crayfish» (PDF). Science. 271 (5247): 366–369. Bibcode:1996Sci…271..366Y. CiteSeerX 10.1.1.470.6528. doi:10.1126/science.271.5247.366. PMID 8553075. S2CID 1575533.
- ^ Fry BG, Roelants K, Champagne DE, Scheib H, Tyndall JD, King GF, et al. (2009). «The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms». Annual Review of Genomics and Human Genetics. Annual Reviews. 10 (1): 483–511. doi:10.1146/annurev.genom.9.081307.164356. PMID 19640225.
- ^ «Serotonin, serotonin receptors and their actions in insects». Neurotransmitter. 2: 1–14. 2015. doi:10.14800/nt.314.
- ^ a b c Schoofs L, De Loof A, Van Hiel MB (31 January 2017). «Neuropeptides as Regulators of Behavior in Insects». Annual Review of Entomology. Annual Reviews. 62 (1): 35–52. doi:10.1146/annurev-ento-031616-035500. ISSN 0066-4170. PMID 27813667.
- ^ a b Wang X, Kang L (7 January 2014). «Molecular mechanisms of phase change in locusts». Annual Review of Entomology. Annual Reviews. 59 (1): 225–244. doi:10.1146/annurev-ento-011613-162019. PMID 24160426.
p. 231,
The change in the number of several potential neurotransmitters … such as serotonin… may play an important role in remodeling the CNS during phase change (26, 56, 80).
p. 233,
In the locust S. gregaria, the amount of serotonin in the thoracic ganglia was positively correlated with the extent of gregarious behavior induced by different periods of crowding. A series of pharmacological and behavioral experiments demonstrated that serotonin plays a key role in inducing initial behavioral gregarization (2, 80). However, serotonin is not responsible for maintaining gregarious behavior because its amount in long-term gregarious locusts is less than half that in long-term solitarious locusts (80). In L. migratoria, the injection of serotonin can also slightly initiate gregarious behavior, but serotonin when accompanying crowding treatment induced more solitarious-like behavior than did serotonin injection alone (48). Significant differences in serotonin levels were not found in brain tissues between the two phases of L. migratoria. A recent report by Tanaka & Nishide (97) measured attraction/avoidance behavior in S. gregaria after single and multiple injections of serotonin at different concentrations. Serotonin had only a short-term effect on the level of some locomotor activities and was not involved in the control of gregarious behavior (97). In addition, it is not clear how the neurotransmitter influences this unique behavior, because a binary logistic regression model used in these studies for the behavioral assay focused mostly on only one behavioral parameter representing an overall phase state. Obviously, behavioral phase change might involve alternative regulatory mechanisms in different locust species. Therefore, these studies demonstrate that CNS regulatory mechanisms governing initiation and maintenance of phase change are species specific and involve the interactions between these neurotransmitters.
Given the key roles of aminergic signaling, what are the downstream pathways involved in the establishment of long-term memory? Ott et al. (63) investigated the role of [] protein kinase[] in the phase change in S. gregaria: … cAMP-dependent protein kinase A (PKA). Through use of pharmacological and RNAi intervention, these authors have demonstrated that PKA… has a critical role in modulating the propensity of locusts to acquire and express gregarious behavior. … Unfortunately, although a correlation between serotonin and PKA was hypothesized, direct evidence was not provided. - ^ a b Zhang L, Lecoq M, Latchininsky A, Hunter D (January 2019). «Locust and Grasshopper Management». Annual Review of Entomology. Annual Reviews. 64 (1): 15–34. doi:10.1146/annurev-ento-011118-112500. PMID 30256665. S2CID 52843907. p. 20:
…gregarization is evoked by… tactile stimulation… Tactile stimuli trigger the increase of biogenic amines, particularly serotonin, in the locust nervous system (1, 116); these amines play critical roles in the neurophysiology of locust behavioral phase change.
- ^ a b Anstey ML, Rogers SM, Ott SR, Burrows M, Simpson SJ (January 2009). «Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts». Science. 323 (5914): 627–630. Bibcode:2009Sci…323..627A. doi:10.1126/science.1165939. PMID 19179529. S2CID 5448884.
- Morgan J (29 January 2009). «Locust swarms ‘high’ on serotonin». BBC News.
- ^ Sitaraman D, LaFerriere H, Birman S, Zars T (June 2012). «Serotonin is critical for rewarded olfactory short-term memory in Drosophila». Journal of Neurogenetics. 26 (2): 238–244. doi:10.3109/01677063.2012.666298. PMID 22436011. S2CID 23639918.
- ^ Bicker G, Menzel R (January 1989). «Chemical codes for the control of behaviour in arthropods». Nature. 337 (6202): 33–39. Bibcode:1989Natur.337…33B. doi:10.1038/337033a0. PMID 2562906. S2CID 223750.
- ^ Cai M, Li Z, Fan F, Huang Q, Shao X, Song G (March 2010). «Design and synthesis of novel insecticides based on the serotonergic ligand 1-[(4-aminophenyl)ethyl]-4-[3-(trifluoromethyl)phenyl]piperazine (PAPP)». Journal of Agricultural and Food Chemistry. 58 (5): 2624–2629. doi:10.1021/jf902640u. PMID 20000410.
- ^ Manahan SE (2002). Toxicological Chemistry and Biochemistry (3rd ed.). CRC Press. p. 393. ISBN 978-1-4200-3212-3.
- ^ Postma TL (2009). «Neurotoxic Animal Poisons and Venoms». In Dobbs MR (ed.). Clinical Neurotoxicology. W.B. Saunders. pp. 463–489. doi:10.1016/B978-032305260-3.50049-6. ISBN 978-0-323-05260-3.
- ^ Gadenne C, Barrozo RB, Anton S (11 March 2016). «Plasticity in Insect Olfaction: To Smell or Not to Smell?». Annual Review of Entomology. Annual Reviews. 61 (1): 317–333. doi:10.1146/annurev-ento-010715-023523. hdl:11336/19586. PMID 26982441. S2CID 207568844.
- ^ Dierick HA, Greenspan RJ (May 2007). «Serotonin and neuropeptide F have opposite modulatory effects on fly aggression». Nature Genetics. 39 (5): 678–682. doi:10.1038/ng2029. PMID 17450142. S2CID 33768246.
- ^ Stoffolano JG, Haselton AT (7 January 2013). «The adult Dipteran crop: a unique and overlooked organ». Annual Review of Entomology. Annual Reviews. 58 (1): 205–225. doi:10.1146/annurev-ento-120811-153653. PMID 23317042.
- ^ a b c d e f Bacqué-Cazenave J, Bharatiya R, Barrière G, Delbecque JP, Bouguiyoud N, Di Giovanni G, et al. (February 2020). «Serotonin in Animal Cognition and Behavior». International Journal of Molecular Sciences. 21 (5): 1649. doi:10.3390/ijms21051649. PMC 7084567. PMID 32121267.
- ^ a b c d e Lucki I (August 1998). «The spectrum of behaviors influenced by serotonin». Biological Psychiatry. 44 (3): 151–162. doi:10.1016/s0006-3223(98)00139-5. PMID 9693387. S2CID 3001666.
- ^ a b c Backström T, Winberg S (25 October 2017). «Serotonin Coordinates Responses to Social Stress-What We Can Learn from Fish». Frontiers in Neuroscience. 11: 595. doi:10.3389/fnins.2017.00595. PMC 5669303. PMID 29163002.
- ^ Berger M, Gray JA, Roth BL (1 February 2009). «The expanded biology of serotonin». Annual Review of Medicine. 60 (1): 355–366. doi:10.1146/annurev.med.60.042307.110802. PMC 5864293. PMID 19630576.
- ^ Alberghina D, Piccione G, Pumilia G, Gioè M, Rizzo M, Raffo P, Panzera M (July 2019). «Daily fluctuation of urine serotonin and cortisol in healthy shelter dogs and influence of intraspecific social exposure». Physiology & Behavior. 206: 1–6. doi:10.1016/j.physbeh.2019.03.016. PMID 30898540. S2CID 81965422.
- ^ Riggio G, Mariti C, Sergi V, Diverio S, Gazzano A (December 2020). «Serotonin and Tryptophan Serum Concentrations in Shelter Dogs Showing Different Behavioural Responses to a Potentially Stressful Procedure». Veterinary Sciences. 8 (1): 1. doi:10.3390/vetsci8010001. PMC 7824451. PMID 33374183.
- ^ Hydbring-Sandberg E, Larsson E, Madej A, Höglund OV (July 2021). «Short-term effect of ovariohysterectomy on urine serotonin, cortisol, testosterone and progesterone in bitches». BMC Research Notes. 14 (1): 265. doi:10.1186/s13104-021-05680-y. PMC 8272283. PMID 34246304.
- ^ a b c Winberg S, Thörnqvist PO (June 2016). «Role of brain serotonin in modulating fish behavior». Current Zoology. 62 (3): 317–323. doi:10.1093/cz/zow037. PMC 5804243. PMID 29491919.
- ^ a b c d Olivier B, Mos J, van der Heyden J, Hartog J (1 February 1989). «Serotonergic modulation of social interactions in isolated male mice». Psychopharmacology. 97 (2): 154–156. doi:10.1007/BF00442239. PMID 2498921. S2CID 37170174.
- ^ Huber R, Smith K, Delago A, Isaksson K, Kravitz EA (May 1997). «Serotonin and aggressive motivation in crustaceans: altering the decision to retreat». Proceedings of the National Academy of Sciences of the United States of America. 94 (11): 5939–5942. Bibcode:1997PNAS…94.5939H. doi:10.1073/pnas.94.11.5939. PMC 20885. PMID 9159179.
- ^ Sanchez CL, Biskup CS, Herpertz S, Gaber TJ, Kuhn CM, Hood SH, Zepf FD (May 2015). «The Role of Serotonin (5-HT) in Behavioral Control: Findings from Animal Research and Clinical Implications». The International Journal of Neuropsychopharmacology. 18 (10): pyv050. doi:10.1093/ijnp/pyv050. PMC 4648158. PMID 25991656.
- ^ a b c d Petersen CL, Hurley LM (October 2017). «Putting it in Context: Linking Auditory Processing with Social Behavior Circuits in the Vertebrate Brain». Integrative and Comparative Biology. 57 (4): 865–877. doi:10.1093/icb/icx055. PMC 6251620. PMID 28985384.
- ^ Loer CM, Kenyon CJ (December 1993). «Serotonin-deficient mutants and male mating behavior in the nematode Caenorhabditis elegans». The Journal of Neuroscience. 13 (12): 5407–5417. doi:10.1523/JNEUROSCI.13-12-05407.1993. PMC 6576401. PMID 8254383.
- ^ Lipton J, Kleemann G, Ghosh R, et al. (August 2004). «Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate». The Journal of Neuroscience. 24 (34): 7427–7434. doi:10.1523/JNEUROSCI.1746-04.2004. PMC 6729642. PMID 15329389.
- ^ a b Murakami H, Murakami S (August 2007). «Serotonin receptors antagonistically modulate Caenorhabditis elegans longevity». Aging Cell. 6 (4): 483–488. doi:10.1111/j.1474-9726.2007.00303.x. PMID 17559503. S2CID 8345654.
- ^ Kaplan DD, Zimmermann G, Suyama K, et al. (July 2008). «A nucleostemin family GTPase, NS3, acts in serotonergic neurons to regulate insulin signaling and control body size». Genes & Development. 22 (14): 1877–1893. doi:10.1101/gad.1670508. PMC 2492735. PMID 18628395.
- ^ Ruaud AF, Thummel CS (July 2008). «Serotonin and insulin signaling team up to control growth in Drosophila». Genes & Development. 22 (14): 1851–1855. doi:10.1101/gad.1700708. PMC 2735276. PMID 18628391.
- ^ Davidson S, Prokonov D, Taler M, et al. (February 2009). «Effect of exposure to selective serotonin reuptake inhibitors in utero on fetal growth: potential role for the IGF-I and HPA axes». Pediatric Research. 65 (2): 236–241. doi:10.1203/PDR.0b013e318193594a. PMID 19262294.
- ^ Ben Arous J, Laffont S, Chatenay D (October 2009). Brezina V (ed.). «Molecular and sensory basis of a food related two-state behavior in C. elegans». PLOS ONE. 4 (10): e7584. Bibcode:2009PLoSO…4.7584B. doi:10.1371/journal.pone.0007584. PMC 2762077. PMID 19851507.
- ^ Sze JY, Victor M, Loer C, Shi Y, Ruvkun G (February 2000). «Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant». Nature. 403 (6769): 560–564. Bibcode:2000Natur.403..560S. doi:10.1038/35000609. PMID 10676966. S2CID 4394553.
- ^ Murakami H, Bessinger K, Hellmann J, Murakami S (July 2008). «Manipulation of serotonin signal suppresses early phase of behavioral aging in Caenorhabditis elegans». Neurobiology of Aging. 29 (7): 1093–1100. doi:10.1016/j.neurobiolaging.2007.01.013. PMID 17336425. S2CID 37671716.
- ^ Côté F, Thévenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, et al. (November 2003). «Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function». Proceedings of the National Academy of Sciences of the United States of America. 100 (23): 13525–13530. Bibcode:2003PNAS..10013525C. doi:10.1073/pnas.2233056100. PMC 263847. PMID 14597720.
- ^ Alarcón J, Cid E, Lillo L, Céspedesa C, Aguila S, Alderete JB (2008). «Biotransformation of indole derivatives by mycelial cultures». Zeitschrift für Naturforschung C. 63 (1–2): 82–84. doi:10.1515/znc-2008-1-215. PMID 18386493. S2CID 29472174.
- ^ Sismaet HJ, Goluch ED (June 2018). «Electrochemical Probes of Microbial Community Behavior». Annual Review of Analytical Chemistry. Annual Reviews. 11 (1): 441–461. Bibcode:2018ARAC…11..441S. doi:10.1146/annurev-anchem-061417-125627. PMID 29490192. S2CID 3632265. p. 449:
Table 1 The respective potential peaks for various electroactive biomolecules that are produced or consumed by microbes reported in the literaturea … Serotonin | Indium tin oxide | +0.67 | 66
- ^ Henson JM, Butler MJ, Day AW (1999). «The Dark Side of the Mycelium: Melanins of Phytopathogenic Fungi». Annual Review of Phytopathology. Annual Reviews. 37 (1): 447–471. doi:10.1146/annurev.phyto.37.1.447. PMID 11701831.
- ^ Anthony M (1984). «Serotonin antagonists». Aust N Z J Med. 14 (6): 888–895. doi:10.1111/j.1445-5994.1984.tb03802.x. PMID 6398056. S2CID 28327178.
- ^ Erspamer V (1954). «Pharmacology of indole-alkylamines». Pharmacol Rev. 6 (4): 425–487. PMID 13236482.
- ^ Negri L (2006). «[Vittorio Erspamer (1909–1999)]». Medicina Nei Secoli. 18 (1): 97–113. PMID 17526278.
- ^ Rapport MM, Green AA, Page IH (December 1948). «Serum vasoconstrictor, serotonin; isolation and characterization». The Journal of Biological Chemistry. 176 (3): 1243–1251. doi:10.1016/S0021-9258(18)57137-4. PMID 18100415.
- ^ Feldberg W, Toh CC (February 1953). «Distribution of 5-hydroxytryptamine (serotonin, enteramine) in the wall of the digestive tract». The Journal of Physiology. 119 (2–3): 352–362. doi:10.1113/jphysiol.1953.sp004850. PMC 1392800. PMID 13035756.
- ^ SciFinder – Serotonin Substance Detail. Accessed (4 November 2012).[full citation needed]
- ^ Twarog BM, Page IH (October 1953). «Serotonin content of some mammalian tissues and urine and a method for its determination». The American Journal of Physiology. 175 (1): 157–161. doi:10.1152/ajplegacy.1953.175.1.157. PMID 13114371.
- ^ Page IH (1 July 1954). «Serotonin (5-Hydroxytryptamine)». Physiological Reviews. American Physiological Society. 34 (3): 563–588. doi:10.1152/physrev.1954.34.3.563. ISSN 0031-9333. PMID 13185755.
Further reading[edit]
- Gutknecht L, Jacob C, Strobel A, Kriegebaum C, Müller J, Zeng Y, et al. (June 2007). «Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation». The International Journal of Neuropsychopharmacology. 10 (3): 309–320. doi:10.1017/S1461145706007437. PMID 17176492.
External links[edit]
Wikimedia Commons has media related to Serotonin.
- 5-Hydroxytryptamine MS Spectrum
- Serotonin bound to proteins in the PDB
- PsychoTropicalResearch Extensive reviews on serotonergic drugs and Serotonin Syndrome.
- Molecule of the Month: Serotonin at University of Bristol
- 60-Second Psych: No Fair! My Serotonin Level Is Low, Scientific American
- Serotonin Test Interpretation on ClinLab Navigator.
Серотонин
(Serotoninum)
Содержание
- Структурная формула
- Русское название
- Английское название
- Латинское название вещества Серотонин
- Химическое название
- Брутто формула
- Фармакологическая группа вещества Серотонин
- Нозологическая классификация
- Код CAS
- Торговые названия с действующим веществом Серотонин
Структурная формула
Русское название
Серотонин
Английское название
Serotonin
Латинское название вещества Серотонин
Serotoninum (род. Serotonini)
Химическое название
5-Окситриптамин (и в виде адипината)
Фармакологическая группа вещества Серотонин
Торговые названия с действующим веществом Серотонин
Наш сайт использует файлы cookie, чтобы улучшить работу сайта, повысить его эффективность и удобство. Продолжая использовать сайт rlsnet.ru, вы соглашаетесь с условиями использования файлов cookie.
Серотонин: гормон хорошего настроения
Рассказываем, почему он так важен для нашего организма и какая пища активизирует его выработку.
Серотонин – химическое вещество, которое обеспечивает передачу сигналов между нервными клетками. Он присутствует в головном мозге, клетках крови (тромбоцитах) и кишечнике. Серотонин регулирует моторику гладких мышечных волокон. То есть именно благодаря ему сокращается, например, ЖКТ или матка при родах.
Серотонин в головном мозге выполняет успокаивающую функцию. У нас в организме все время соблюдается определенный баланс между бодрствованием и сном, результатом которого является наше общее состояние. Так вот серотонин «играет» на стороне сна.
Еще одна важная функция – контроль уровня болевой чувствительности. Мы все по-разному переносим боль. Люди, которым это удается легче, обладают активной серотониновой системой.
Кроме того, важная задача серотонина – помогать нам сосредоточиться на главном. В мозгу постоянно идет огромное количество сенсорных, эмоциональных и информационных потоков, и серотонин отсекает лишнее. Если ему мешать, начнется путаница.
И конечно, серотонин играет важную роль в поддержании эмоционального состояния: он подавляет отрицательные эмоции и не дает нам уходить в депрессивное состояние. Нарушение баланса между центрами положительных и отрицательных эмоций становится причиной тяжелой формы депрессии, которая, в свою очередь, может привести к суициду. В этом случае человеку в большинстве случаев необходимы антидепрессанты.
Таким образом, нехватка серотонина вызывает нарушение сна, тревожность и агрессию, ухудшает память.
Повысить уровень серотонина можно несколькими способами. Один из самых простых –– принимать солнечные ванны. Солнечный свет стимулирует выработку этого вещества. Также можно уделить больше внимания физической активности. И конечно, нужно правильно питаться. Дело в том, что серотонин возникает при превращениях триптофана, пищевой аминокислоты, которую наш организм сам не синтезирует. Получить ее мы можем только с едой. Средняя суточная норма триптофана – 250 мг.
Продукты, в которых содержится триптофан
- Лидирующую позицию по содержанию триптофана занимает сыр. Например, в «Швейцарском» (50% жирности) на 100 г продукта приходится 1000 мг триптофана – это 400% от суточной нормы. Почти также много этой аминокислоты в «Рокфоре», «Чеддере», «Пошехонском». Чуть меньше – в «Пармезане», «Фете», брынзе, молоке и других молочных и кисломолочных продуктах (йогурт, кефир, сливки, творог).
- Яйца: куриные и перепелиные.
- Триптофан есть в разных видах мяса и птицы. Среди них – индейка, курица, говядина, баранина, свинина.
- В рацион для хорошего настроения нужно включить рыбу и морепродукты: горбушу, морского окуня, сельдь, скумбрию, ставриду, судака, треску, щуку, кету, икру, кальмаров и другие.
- Крупы: гречиха, манка, кукурузная, овсяная, перловая, пшеничная, рисовая.
- Орехи и семена: арахис, грецкий орех, кедровый орех, миндаль, кунжут, семена подсолнечника, фисташки, фундук.
- Бобовые: горох, фасоль, чечевица.
- Грибы: белые, шиитаке, вешенки.
Еще один важный момент: чтобы достичь головного мозга и поучаствовать в синтезе серотонина, триптофану нужны углеводы. Поэтому составляйте свой рацион так, чтобы в нем были соответствующие продукты.
Питайтесь правильно, соблюдайте баланс, и хорошее настроение вам будет обеспечено.
Факт: положительные эмоции невозможны без участия гормонов. Стресс, депрессия, тоска и прочие негативные явления зачастую возникают из-за того, что блокируется выработка дофамина, серотонина, окситоцина и эндорфинов. Эндокринолог Ольга Пенкрат объясняет, что нужно делать, чтобы «подстегнуть» выработку «гормонов счастья», и чего избегать, дабы не впасть в депрессию.
Ольга Пенкрат
врач-эндокринолог первой категории медицинского центра «Мед-Практика»
Дофамин
Что это?
— Дофамин — биологически активное вещество, которое вырабатывается в мозгу синапсами нейронов и служит для передачи нервных импульсов. Кроме того, участвуя в регуляции сердечно-сосудистой системы, оно синтезируется в надпочечниках, почках и кишечнике. Это одно и то же химическое соединение, однако дофамин, синтезированный вне центральной нервной системы, в головной мозг не попадает и, соответственно, влияния на передачу нервных импульсов не оказывает.
Роль дофамина в организме
Как гормон:
- повышает артериальное давление, частоту и силу сердечных сокращений;
- расслабляет гладкую мускулатуру желудка и кишечника;
- увеличивает фильтрацию жидкости, кровоток в почках, ускоряет выделение натрия с мочой.
Как нейромедиатор оказывает влияние на:
- формирование мотивации;
- чувство удовольствия;
- ощущение награды и желания;
- эмоциональные реакции, сопровождающие двигательную активность.
Выделению дофамина способствует:
- Достижение цели и даже само предвкушение этого момента. Одни лишь мысли о триумфе вызывают выброс нейромедиатора. Когда же цель достигнута, выработка дофамина снижается.
- Чувство любви. Вместе с гормоном окситоцином дофамин помогает формировать чувство привязанности, в том числе материнское. Кроме того, он обеспечивает возможность эффективно учиться на своих ошибках, а нехватка дофамина может приводить к игнорированию негативного опыта.
- Приятные тактильные ощущения. Дофамин естественным образом вырабатывается в больших количествах во время интимной близости.
- Употребление любимой пищи. Употребление всеми любимого шоколада повышает уровень дофамина. Однако своеобразными «кирпичиками», предшественниками дофамина, служат молекулы аминокислоты тирозина. Чем больше его содержание в продуктах, тем выше шанс получить удовольствие. Тирозин содержится, к примеру, в мясе, бобовых (соя, чечевица, фасоль), орехах, сыре и твороге.
- Отдых и физическая активность. Отличный помощник в выработке дофамина — полноценный сон. Второй вариант противоположен — это любая активность, будь то занятие определенныи видом спорта или обычная пробежка. Дофамин призван мотивировать человека, который ждет какого-то вознаграждения в конце тренировки: похудения, отдыха и т.д. А за это предвкушение тоже отвечает дофамин.
Будьте осторожны. В обмене дофамина могут принимать участие некоторые кардиологические и психиатрические медицинские препараты. Шутить с этим процессом нельзя, обязательно посоветуйтесь с врачом.
Кстати
— Дофамин — не всегда синоним позитива, как многие привыкли считать. Он помогает организму адаптироваться к стрессовым ситуациям, выделяется при травмах и болевом синдроме.
Что тормозит выработку дофамина?
— Выработку и высвобождение дофамина в мозге в 5-10 раз увеличивают наркотические вещества. А затем — уменьшают, заставляя повышать дозу. Чувство удовольствия вырабатывается искусственным образом.
Если человек продолжает так себя «поощрять», постепенно мозг адаптируется к искусственно повышенному уровню дофамина, при этом сам производит его в меньших количествах. Это побуждает наркомана увеличивать дозу, чтобы вернуть приятные ощущения, ведь эйфория вдруг сменяется подавленностью и депрессией.
Блокирует выработку нейромедиатора также и злоупотребление жирной и сладкой пищей. По той же схеме, что и с наркотиками: организм требует еще и еще, что в итоге приводит к перееданию и пищевой зависимости.
Серотонин
Что это?
— Как и дофамин, серотонин является нейромедиатором и гормоном. 95% этого вещества вырабатывается слизистой оболочкой кишечника и лишь 5% — в головном мозге.
Роль серотонина в организме:
- улучшает память, внимание, восприятие;
- ускоряет и облегчает движения;
- снижает болевой порог;
- контролирует либидо и репродуктивную функцию;
- обеспечивает полноценный сон;
- помогает пищеварению;
- уменьшает аллергические реакции;
- регулирует сокращение матки и маточных труб во время родов;
- способствует хорошему настроению;
- участвует в синтезе гормонов гипофиза.
Выделению серотонина способствуют:
- Триптофан и глюкоза. Триптофан — это аминокислота, из которой образуется серотонин. Глюкоза помогает триптофану добраться к мозгу для выработки серотонина. Какая пища богата триптофаном? Это молочные продукты (особенно сыр), финики, сливы, инжир, томаты, соя и черный шоколад. Глюкозы много во фруктах, овощах, ягодах и меде.
- Магний. Он способствует превращению триптофана в серотонин. Содержится во фруктах, орехах, бобовых и цельных зернах.
- Солнечный свет. Для синтеза серотонина он абсолютно необходим. Витамин D регулирует переход триптофана в серотонин. Большое его количество действительно прибавит вам радости. Но помните: эффект этот необходимо постоянно подкреплять.
Будьте осторожны. Избыток серотонина порой провоцирует развитие серотонинового синдрома. Такое бывает при передозировке антидепрессантов ингибиторного типа. Их действие заключается в повышении уровня серотонина и его задержке в организме. Особенно часто проблема возникает, если человек занимается самолечением или игнорирует рекомендации врача в надежде на то, что увеличенная доза препарата подарит стойкое и сильное ощущение счастья.
Что тормозит выработку серотонина?
- Кофеин. Мало того, что он снижает уровень серотонина, так еще и ухудшает аппетит.
- Алкоголь подавляет синтез серотонина и блокирует функции уже имеющегося в организме гормона. Возникает риск возникновения абстинентного синдрома — физического и/или психического расстройства, развивающегося у больных наркоманией спустя некоторое время после прекращения приема наркотика или уменьшения его дозы (ломка). Противостоять этому синдрому, как правило, получается лишь с помощью очередной порции спиртного. Со временем это приводит к алкогольной зависимости.
Эндорфины
Что это?
— Эндорфины — группа химических соединений, которые естественным путем вырабатываются в нейронах головного мозга.
Роль эндорфинов в организме:
- обезболивающий эффект;
- стрессоустойчивость;
- функция поощрения: организм, благополучно преодолевший опасную для жизни ситуацию, получает поощрение в виде стимуляции центров удовольствия — чувство эйфории;
- участие в регуляции возбуждения и торможения. В первой фазе стресса, когда вопрос жизни и смерти еще не решен, работает та часть эндорфинной системы, которая усиливает продуктивное мышление. После решения вопроса жизни и смерти наступает черед торможения — перехода организма в режим сбережения;
- стимуляция процессов заживления: эндорфины ускоряют процессы регенерации;
- способствуют формированию образного мышления, ассоциаций и творческих фантазий.
Выделению эндорфинов способствуют:
Секс. Это простая и быстрая возможность повышения концентрации эндорфинов. В момент интимной близости в кровь выбрасывается огромное количество «гормонов счастья».
Вкусная еда. Особенно любимая. Хороший обед — это отличный источник неиссякаемого удовольствия.
Позитивное мышление. Даже приятные мечты способны привести человека в состояние блаженства.
Ультрафиолет увеличивает концентрацию эндорфинов в организме
Что тормозит выделение эндорфинов?
— Длительное употребление алкоголя и наркотических веществ. Наличие хронических заболеваний.
Окситоцин
Что это?
— Этот гормон вырабатывается в гипоталамусе, отделе центральной нервной системы. Активное вещество поступает из клеток гипоталамуса в гипофиз, где хранится и выделяется под воздействием внешних стимулов.
Роль окситоцина в организме:
- вызывает эмоциональную привязанность;
- обеспечивает стрессоустойчивость;
- способствует сокращению гладкой мускулатуры матки, помогая прохождению плода по родовым путям. Регулируя мускулатуру млечных протоков женской груди, обеспечивает здоровую лактацию.
Выделению окситоцина способствуют:
- Прикосновения и чувство доверия. Любые приятные контакты с родными и близкими (и тактильные, и эмоциональные) вызывают активизацию окситоцина. Хорошим стимулятором для этого процесса становится и релакс-массаж.
- Кормление ребенка грудью. Окситоцин при лактации способствует сокращению мышечного слоя молочной железы и выделению грудного молока из нее. В этом смысле кормящим мамам повезло. Но выделению окситоцина может поспособствовать и просто стимуляция ореолы молочной железы. Даже если у вас нет детей.
- Сильная боль, хроническая боль. Это не самый приятный способ, но он работает. Окситоцин тоже призван справляться с болью. Возможно, поэтому некоторым людям нравится причинять себе боль.
- Так же, как и в случае с перечисленными гормонами, на повышение уровня окситоцина может повлиять: любимая пища, оргазм, физические упражнения и хорошее расположение духа.
Что тормозит выработку окситоцина?
— Этому способствует в первую очередь алкоголь, а также отсутствие любви, дружбы, депрессия и изоляция от людей.
Как видите, повлиять на собственные эмоции вполне реально благодаря питанию, физическим нагрузкам, позитивному образу жизни и так далее. Главное — делать это разумно, без вреда для организма, и не переусердствовать. Учитесь не зацикливаться на проблемах и каждый день открывать для себя что-то новое. Это лучший способ естественно и безопасно для здоровья повысить уровень гормонов счастья и чувствовать себя на все сто! Важно также понимать, что большого клинического значения уровень данных биологически активных веществ не имеет, лабораторно в рутинной практике он не определяется. А вот при таких проблемах, как гормональная андрогения, гиперандрогения, сбои менструального цикла, анализ на определенную группу гормонов просто необходим.
При создании материала частично была использована информация из книги «Регуляторные системы организма человека» В.А. Дубинина.
Читайте также:
То вверх, то вниз! Врач рассказывает о признаках гормонального дисбаланса
Акне, избыточный рост волос, облысение… Чем опасна гиперандрогения, и почему ее нельзя игнорировать?
- Как научиться управлять серотонином?
- Что случится, если уровень серотонина нарушится?
- Но стоит ли сразу пускать в ход «тяжелую артиллерию»?
- Как работают витамины для хорошего настроения?
- Управлять гормонами счастья легко!
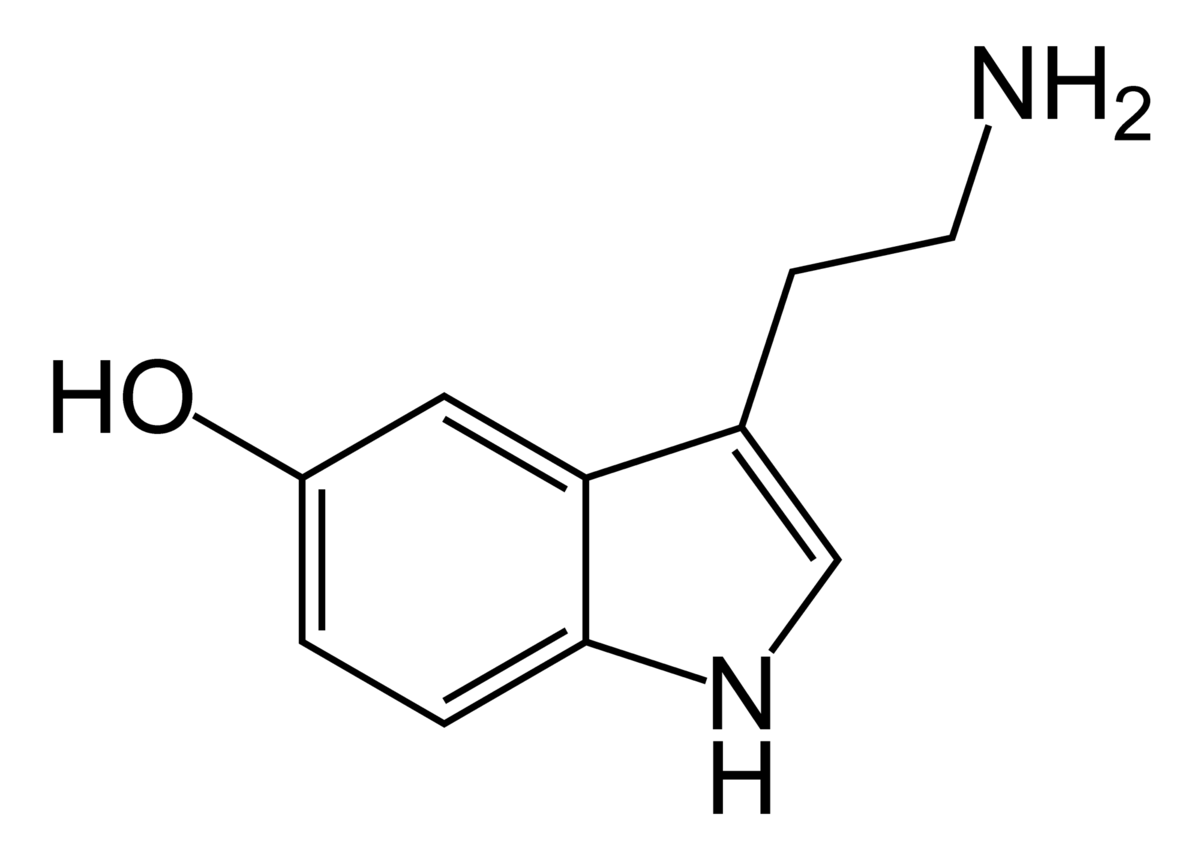
- серотонин – его называют гормоном счастья, который дарит чувство эйфории;
- дофамин – он отвечает за наслаждение и закрепляет положительный опыт;
- окситоцин – он рождает такие чувства, как любовь, нежность и привязанность.
Чаще всего эта «троица» работает в связке, формируя все наши позитивные эмоции. Но сейчас мы будет говорить именно о серотонине. Многие люди уже знают, как называется этот гормон радости и счастья. Но далеко не все понимают, как он работает. И еще меньше людей знают, как регулировать уровень этого гормона в организме.
Как научиться управлять серотонином?
Этот нейромедиатор, регулирующий наше с вами настроение, не так прост, как может показаться на первый взгляд. Наверняка вы думаете, что серотонин – еще один гормон, который вырабатывается в человеческом мозге. Это так, но лишь отчасти. Вот вам сухие факты: 95% серотонина вырабатывается в кишечнике. И только 5% — в мозге.
Так неужели путь к нашему счастью лежит через желудок? И это не совсем так. Серотонин – сложное химическое вещество, производная аминокислоты триптофана и, по совместительству, менеджер нашего мозга. Этот гормон отвечает за обширный ряд процессов как в самом мозге, так и во всем организме человека:
- Серотонин в мозге помогает нервным клеткам лучше взаимодействовать.
- Гормон радости влияет на сон и аппетит, состояние мышц и работу организма, характер и настроение.
- Важнейший нейромедиатор оказывает воздействие на когнитивные функции (внимание, память, общение и пр.).
- Серотонин отвечает за нормальную свертываемость крови и стабильную работу кровеносной системы.
- Гормон радости помогает нам испытывать интерес и влечение к противоположному полу.
Что случится, если уровень серотонина нарушится?
Очень часто мы слышим о том, как низкий уровень гормона счастья – это плохо. Но организму человека вредит не только недостаток, но и избыток серотонина. Другими словами, любые нарушения выработки этого нейромедиатора провоцируют целый список негативных последствий и состояний:
- плохое настроение и хроническую депрессию;
- резкое снижение сексуального влечения;
- проблемы с пищеварением и сном;
- тягу к сладкому и набор веса;
- понижение активности.
Перечисленные проблемы – лишь вершина айсберга. Работа серотонина, основанная на двух веществах – аминокислоте триптофан и ее производном 5-гидрокситриптофан, очень сложная. Иногда гормональный дисбаланс настолько выражен, что специалистам не остается ничего другого, как прописать антидепрессанты – ингибиторы обратного захвата серотонина.
Читайте также: Как бороться со стрессом на работе?
Но стоит ли сразу пускать в ход «тяжелую артиллерию»?
Мы знаем, что антидепрессанты действуют быстро. Но также мы знаем, что у них хватает недостатков, важнейшими из которых являются привыкание и различные побочные эффекты, способные серьезно пошатнуть и без того ослабленное здоровье. На изображении ниже показано, как «жестко работают» антидепрессанты. Стоит задуматься нужны ли такие радикальные меры???
Есть ли безопасная альтернатива таблеткам? Есть. Это правильный образ жизни и умение смотреть на мир позитивно. «Рецепт» вашего счастья будет состоять не из набора лекарств, а из совершенно других компонентов:
- Питание. В здоровой пище есть все, что нужно для выработки гормона серотонина в достаточном количестве. Откажитесь от быстрых углеводов и вредной еды в пользу полезных углеводов и продуктов, содержащих триптофан. Серотонин «растет» на разнообразии: злаках и крупах, овощах и фруктах, яйцах и молочной продукции, нежирном мясе и жирной рыбе, орехах, семенах и сухофруктах.
На изображении показаны продукты богатые триптофаном.
- Движение. Ученые не раз доказывали, что любая физическая активность быстро (буквально за 30-40 минут) повышает уровень серотонина в крови. Танцы, пешие прогулки, бассейн, езда на велосипеде, утренняя пробежка – активностей много, не обязательно идти в спортзал. Но есть одно «но»: активным нужно быть постоянно, а не время от времени.
- Сон и медитация. Счастливый человек умеет вовремя «выключаться» и отпускать негатив из своей жизни. Иногда сделать это сложно. И тогда на помощь приходят медитация и здоровый сон, во время которых уровень гормона счастья также повышается. Приучите себя ложиться и вставать в одно и то же время – и у вас не будет проблем с серотонином.
- Больше солнца. В нашем климате солнечные деньки – нечастое явление. Но даже в пасмурный день наша кожа все равно получает свою порцию ультрафиолета. А, значит, ответ на вопрос «как повысить серотонин» прост: чаще гуляйте. Прогулки подарят организму максимум гормона счастья и полезного витамина D.
- Омега-3. Нам сложно поддерживать оптимальный уровень незаменимых полиненасыщенных жирных кислот, которые в избытке содержатся только в мясе жирной рыбы северных морей. Но сейчас уже есть достойная альтернатива – комплексы БАДов с Омега-3. Такие добавки – настоящее «лакомство» для мозга и профилактика целого ряда заболеваний.
- Удовольствие. Серотонин отвечает за удовольствие и наслаждение. А как получить больше таких эмоций? Идей масса: например, влюбиться, найти новое увлекательное хобби, чаще встречаться с друзьями, больше времени проводить с близкими. Чем больше в вашей жизни будет ярких эмоций и позитива, тем выше будет и уровень гормона счастья.
- «Помощники». К сожалению, даже при правильном питании не удается получить из пищи 100% необходимых для питания мозга и организма веществ. И тогда на помощь приходит «серотонин в таблетках». Это специальные растительные витаминные комплексы, которые помогают гормону счастья вырабатываться правильно и в необходимом объеме.
Как работают витамины для хорошего настроения?
В отличие от антидепрессантов, которые ингибируют обратный захват серотонина, витамины на растительной основе действуют иначе – более мягко. Они приводят все нейромедиаторы в баланс и помогают гормону счастья вырабатываться в нужном количестве, не причиняя серьезного вреда организму человека:
- борются с усталостью и раздражительностью;
- повышают память, внимание и концентрацию;
- устраняют бессонницу и другие нарушения сна;
- защищают от депрессии и нервных расстройств;
- возвращают интерес к жизни и нормализуют аппетит.
Постоянные стрессы на работе и дома не позволяют всегда быть на пике активности. Но теперь эта проблема решается легко: всего одна капсула растительных витаминов в день – и вам уже намного легче справляться с вызовами, которые подготовил для вас этот мир. Забывчивость, рассеянность и апатичность сменятся бодростью и позитивным настроением.
Управлять гормонами счастья легко!
Секрет эффективность растительных витаминов для настроения – в идеальном составе. В них нет ничего лишнего помимо активных компонентов – экстрактов лекарственных трав. Например, это могут быть:
- трава зверобоя и клевера;
- родиола розовая и валериана;
- володушка и кора муиры пуамы.
Такие витамины даже при длительном применении часто оказываются более эффективными и безопасными в сравнении с синтетическими препаратами и антидепрессантами, вызывающими привыкание, эффект отмены, сонливость и другие побочные эффекты.
Теперь вы знаете, как правильно повышать уровень серотонина в организме, чтобы положительный результат не заставил себя ждать. Позвольте гормону счастья вырабатываться в полном объеме – и ваша жизнь станет яркой, а каждый день будет приносить максимум пользы и удовольствия.
| Серотонин | |
|---|---|
 |
|
 |
|
| Общие | |
| Систематическое наименование |
3-(2-аминоэтил)-1H-индол-5-ол |
| Сокращения | 5-HT |
| Традиционные названия |
5-гидрокситриптамин, серотонин, энтерамин, тромбоцитин, 3-(β-аминоэтил)-5-гидроксииндол, тромботонин |
| Хим. формула | N2OC10H12 |
| Физические свойства | |
| Состояние | твёрдое кристаллическое вещество, белого цвета |
| Молярная масса | 176,2151 ± 0,0095 г/моль |
| Термические свойства | |
| Т. плав. | 167,5 °C |
| Т. кип. | 416 ±30,0 °C |
| Химические свойства | |
| pKa | 10,4 |
| Растворимость в воде | 20 г/100 мл |
| Структура | |
| Дипольный момент | 2,98 Д |
| Классификация | |
| Рег. номер CAS | 50-67-9 |
| PubChem | 5202 |
| Рег. номер EINECS | 200-058-9 |
| SMILES |
NCCc1c[nH]c2ccc(O)cc12 |
| InChI |
1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 QZAYGJVTTNCVMB-UHFFFAOYSA-N |
| ChEBI | 28790 |
| ChemSpider | 5013 |
| Безопасность | |
| ЛД50 |
60 мг/кг (мыши, перорально), 81 мг/кг (мыши, внутривенно), 601 мг/кг (мыши, подкожно), 750 мг/кг (крысы, подкожно), 4500 мг/кг (крысы, внутрибрюшинно), 13 мг/кг (морские свинки, внутривенно), 5 мг/кг (кошка, внутривенно) |
| Токсичность |
высокотоксичен для мелких животных (птиц, млекопитающих),
|
| Приводятся данные для стандартных условий (25 °C, 100 кПа), если не указано иного. |
Серотони́н, 5-гидрокситриптамин, 5-НТ — один из основных нейромедиаторов. По химическому строению серотонин относится к биогенным аминам, классу триптаминов. Серотонин часто называют «гормоном хорошего настроения» и «гормоном счастья»[1].
Содержание
- 1 История
- 2 Биосинтез
- 2.1 Влияние солнечного света
- 3 Рецепторы серотонина
- 4 Метаболизм (анаболизм и катаболизм) серотонина
- 5 Серотонин и норадреналин
- 6 Физиологическая роль
- 6.1 Серотонин как нейромедиатор
- 6.1.1 Расположение нейронов
- 6.1.2 «Круговорот» серотонина
- 6.1.3 Функции серотонина
- 6.2 Серотониновый синдром
- 6.3 Серотонин как гормон
- 6.3.1 Повышение свёртываемости крови
- 6.3.2 Влияние на аллергические и воспалительные реакции
- 6.3.3 Влияние на пищеварение
- 6.3.4 Влияние на процессы в матке
- 6.3.5 Влияние на половую систему
- 6.3.6 Один из гормонов удовольствия
- 6.3.7 Изменение уровня серотонина
- 6.1 Серотонин как нейромедиатор
- 7 Патологии, связанные с серотонином
- 8 Пищевые продукты
- 9 См. также
- 10 Примечания
- 11 Литература
- 12 Ссылки
История[править | править код]
В 1935 году итальянским фармакологом Витторио Эрспамером впервые было выделено вещество из слизистой желудочно-кишечного тракта, сокращающее гладкую мускулатуру. Некоторые считали, что это был всего лишь адреналин, но только через два года первооткрывателю удалось доказать, что этим веществом оказался ранее неизвестный амин. Эрспамер назвал полученное соединение «энтерамином»[2]. В 1948 году Морис Раппорт, Арда Грин и Ирвин Пейдж в Кливлендской клинике обнаружили сосудосуживающее вещество в сыворотке крови, которое назвали «серотонином». Структура данного вещества, предложенная Морисом Раппортом, в 1951 году была подтверждена химическим синтезом. В 1952 году было доказано, что энтерамин и серотонин — одно и то же вещество[3]. В 1953 году нейрофизиологам Ирвину Пейджу и Бетти Твэрег удалось обнаружить серотонин в головном мозге[4].
После открытия серотонина началось изучение его рецепторов. В 1957 Джон Гаддум провёл ряд исследований, по итогам которых выяснилось, что серотониновые рецепторы неоднородны: способность серотонина сокращать гладкие мышцы блокировалась диэтиламидом Д-лизергиновой кислоты (ЛСД — мощный галлюциноген и психотропный препарат вёл себя как агонист серотонина в периферических тканях), а свойство возбуждать вегетативные нервные узлы предотвращалось морфином. Соответствующие рецепторы были названы «Д»- и «М»-серотониновыми рецепторами. В 90-х годах XX века с помощью методов молекулярной биологии удалось выяснить, что существуют, по крайней мере, 14 видов серотониновых рецепторов, которые отвечают за разнообразные функции серотонина.
Биосинтез[править | править код]
Серотонин образуется из аминокислоты триптофана путём её последовательного 5-гидроксилирования ферментом 5-триптофангидроксилазой (в результате чего получается 5-гидрокситриптофан, 5-ГТ) и затем декарбоксилирования получившегося гидрокситриптофана ферментом триптофандекарбоксилазой. 5-триптофангидроксилаза синтезируется только в соме серотонинергических нейронов, гидроксилирование происходит в присутствии ионов железа и кофактора птеридина.
Влияние солнечного света[править | править код]
Для синтеза серотонина абсолютно необходим солнечный свет. Именно поэтому в солнечные дни человек пребывает в хорошем настроении. Этим же процессом можно объяснить и общеизвестную зимнюю депрессию[5][неавторитетный источник?].
В работе Ронда Патрика и Брюса Эймса сказано, что Витамин D регулирует переход триптофана в серотонин и взаимодействует с генетическими серотониновыми путями[6][неавторитетный источник?].
![{displaystyle {ce {{text{триптофан}}->[{ce {{text{витамин}}D}}]{text{серотонин}}}}}](https://web.archive.org/web/20190531062311im_/https://wikimedia.org/api/rest_v1/media/math/render/svg/fbcb72ef9f519edf40adbb93c31494eba2c88e75)
Рецепторы серотонина[править | править код]
Рецепторы серотонина представлены как метаботропными, так и ионотропными. Всего насчитывается семь типов таких рецепторов, 5-HT 1-7, причём 5-HT3-рецептор — ионотропный, остальные — метаботропные, семидоменные, связанные с G-белками. Установлено сходство метаботропных 5-HT рецепторов с рецепторами норадреналина.
5-HT1 тип, насчитывающий несколько подтипов: 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, которые могут быть как пре-, так и постсинаптическими, подавляет аденилатциклазу; 5-HT4 и 5-HT7 — стимулируют; 5-HT2, насчитывающий несколько подтипов: 5-HT2А, 5-HT2B, 5-HT2C, которые могут быть только постсинаптическими, активирует инозитолтрифосфат. 5-HT5 также подавляет аденилатциклазу[7].
Для некоторых типов рецепторов обнаружены эндогенные лиганды, помимо серотонина. Это, например, 5HT-модулин (Leu-Ser-Ala-Leu), эндогенный лиганд 1B и 1D пресинаптических рецепторов, индуктор тревожности и стресса.
Структура серотонина имеет сходство со структурой психоактивного вещества ЛСД. ЛСД действует как агонист некоторых 5-HT рецепторов и ингибирует обратный захват серотонина, увеличивая его содержание.
Метаболизм (анаболизм и катаболизм) серотонина[править | править код]
Под действием фермента моноаминооксидазы (МАО) серотонин превращается в 5-гидроксииндолальдегид, который, в свою очередь, может обратимо превращаться в 5-гидрокситриптофол под действием алкогольдегидрогеназы. Необратимо 5-гидроксииндолальдегид под действием ацетальдегиддегидрогеназы превращается в 5-гидроксииндолуксусную кислоту, которая затем выводится с мочой и калом.
Серотонин является предшественником мелатонина, образующегося под действием фермента эпифиза ААНАТ в эпифизе.
Также, превращаясь с помощью МАО в 5-гидроксииндол-3-ацетальдегид, он может под действием альдегидредуктазы превратиться в триптофол, а под действием ацетальдегидрогеназы-2 — в оксииндолуксусную кислоту (5-HIAA).
Серотонин может принимать участие в формировании эндогенных опиатов, вступая в реакцию с ацетальдегидом с образованием гармалола.
Серотонин и норадреналин[править | править код]
Существует определённое сходство в строении клеточных рецепторов к серотонину и норадреналину, подобие их транспортных клеточных систем. Известно также, что норадреналин ингибирует выброс серотонина. На их связи основано действие антидепрессанта миртазапина, который, блокируя альфа-2 рецепторы норадреналина, по принципу отрицательной обратной связи повышает содержание в синаптической щели и норадреналина, и серотонина (так как его ингибирование также тормозится) до нормы.
Физиологическая роль[править | править код]
Физиологические функции серотонина чрезвычайно многообразны. Серотонин «руководит» очень многими функциями в организме. Например, очень интересны исследования его влияния на проявление боли.
Доктором Виллисом доказано, что при снижении серотонина повышается чувствительность болевой системы организма, то есть даже самое слабое раздражение отзывается сильной болью.
Серотонин как нейромедиатор[править | править код]
Расположение нейронов[править | править код]
Серотонин играет роль нейромедиатора в центральной нервной системе. Серотонинергические нейроны группируются в стволе мозга: в варолиевом мосту и ядрах шва. От моста идут нисходящие проекции в спинной мозг, нейроны ядер шва дают восходящие проекции к мозжечку, лимбической системе, базальным ганглиям, коре. При этом нейроны дорсального и медиального ядер шва дают аксоны, различающиеся морфологически, электрофизиологически, мишенями иннервации и чувствительностью к некоторым нейротоксичным агентам, например, метамфетамину.
«Круговорот» серотонина[править | править код]
Синтезированный нейроном серотонин закачивается в везикулы. Этот процесс является протон-сопряжённым транспортом. В везикулу с помощью протон-зависимой АТФазы закачиваются ионы H+. При выходе протонов по градиенту в везикулу поступают молекулы серотонина.
Далее, в ответ на деполяризацию терминали, серотонин выводится в синаптическую щель. Часть его участвует в передаче нервного импульса, воздействуя на клеточные рецепторы постсинаптической мембраны, а часть возвращается в пресинаптический нейрон с помощью обратного захвата. Ауторегуляция выхода серотонина обеспечивается путём активации пресинаптических 5-НТ рецепторов, запускающих каскад реакций, которые регулируют вход ионов кальция внутрь пресинаптической терминали. Ионы кальция, в свою очередь, активируют фосфорилирование фермента 5-триптофангидроксилазы, обеспечивающей превращение триптофана в серотонин, что приводит к усилению синтеза серотонина.
Обратный захват производится транспортером серотонина, двенадцатидоменным белком, производящим натрий-калий-сопряжённый транспорт. Вернувшийся в клетку медиатор расщепляется с помощью моноаминооксидазы до 5-гидроксилиндолилуксусной кислоты.
Химизм транспортных систем серотонина также подобен таковым норадреналина.
Функции серотонина[править | править код]
Серотонин облегчает двигательную активность, благодаря усилению секреции субстанции Р в окончаниях сенсорных нейронов путём воздействия на ионотропные и метаботропные рецепторы.
Серотонин наряду с дофамином играет важную роль в механизмах гипоталамической регуляции гормональной функции гипофиза. Стимуляция серотонинергических путей, связывающих гипоталамус с гипофизом, вызывает увеличение секреции пролактина и некоторых других гормонов передней доли гипофиза — действие, противоположное эффектам стимуляции дофаминергических путей.
Серотонин также участвует в регуляции сосудистого тонуса.
Серотониновый синдром[править | править код]
Избыток серотонина может быть потенциально опасен, вызывая последствия, известные как серотониновый синдром. Такая критическая концентрация серотонина зачастую является следствием параллельного применения антидепрессантов классов ингибиторов моноаминооксидазы и селективных ингибиторов обратного захвата серотонина[8].
Серотонин как гормон[править | править код]
Повышение свёртываемости крови[править | править код]
Серотонин играет важную роль в процессах свёртывания крови. Тромбоциты крови содержат значительные количества серотонина и обладают способностью захватывать и накапливать серотонин из плазмы крови. Серотонин повышает функциональную активность тромбоцитов и их склонность к агрегации и образованию тромбов. Стимулируя специфические серотониновые рецепторы в печени, серотонин вызывает увеличение синтеза печенью факторов свёртывания крови. Выделение серотонина из повреждённых тканей является одним из механизмов обеспечения свёртывания крови по месту повреждения.
Влияние на аллергические и воспалительные реакции[править | править код]
Серотонин участвует в процессах аллергии и воспаления. Он повышает проницаемость сосудов, усиливает хемотаксис и миграцию лейкоцитов в очаг воспаления, увеличивает содержание эозинофилов в крови, усиливает дегрануляцию тучных клеток и высвобождение других медиаторов аллергии и воспаления.
Местное (например, внутримышечное) введение экзогенного серотонина вызывает сильную боль в месте введения. Предположительно серотонин наряду с гистамином и простагландинами, раздражая рецепторы в тканях, играет роль в возникновении болевой импульсации из места повреждения или воспаления.
Влияние на пищеварение[править | править код]
Также большое количество серотонина производится в кишечнике. Серотонин играет важную роль в регуляции моторики и секреции в желудочно-кишечном тракте, усиливая его перистальтику и секреторную активность. Кроме того, серотонин играет роль фактора роста для некоторых видов симбиотических микроорганизмов, усиливает бактериальный метаболизм в толстой кишке. Сами бактерии толстой кишки также вносят некоторый вклад в секрецию серотонина кишечником, поскольку многие виды симбиотических бактерий обладают способностью декарбоксилировать триптофан. При дисбактериозе и ряде других заболеваний толстой кишки продукция серотонина кишечником значительно снижается.
Массовое высвобождение серотонина из погибающих клеток слизистой желудка и кишечника при воздействии цитотоксических химиопрепаратов является одной из причин возникновения тошноты и рвоты, диареи при химиотерапии злокачественных опухолей. Аналогичное состояние бывает при некоторых злокачественных опухолях, эктопически продуцирующих серотонин.
Влияние на процессы в матке[править | править код]
Большое содержание серотонина также отмечается в матке. Серотонин играет роль в паракринной регуляции сокращения матки и маточных труб и в координации родов. Продукция серотонина в миометрии возрастает за несколько часов или дней до родов и ещё больше увеличивается непосредственно в процессе родов. Также серотонин вовлечён в процесс овуляции — содержание серотонина (и ряда других биологически активных веществ) в фолликулярной жидкости увеличивается непосредственно перед разрывом фолликула, что, по-видимому, приводит к увеличению внутрифолликулярного давления.
Влияние на половую систему[править | править код]
Серотонин оказывает значительное влияние на процессы возбуждения и торможения в системе половых органов. Например, увеличение концентрации серотонина у мужчин задерживает наступление эякуляции.
Один из гормонов удовольствия[править | править код]
Серотонин часто называют «гормоном счастья», он вырабатывается в организме в моменты экстаза, его уровень повышается во время эйфории и понижается во время депрессии. Для выработки серотонина обязательно нужен ультрафиолет, недостаток ультрафиолета в зимнее время года и является причиной столь распространённой сезонной депрессии.
Изменение уровня серотонина[править | править код]
На уровень серотонина в организме можно влиять:
- с помощью физических упражнений, изменения ритма и глубины дыхания
- диетами
- натуральными и химическими лекарственными препаратами
- солнечным светом
Чтобы вырабатывался серотонин, в организм обязательно должны поступать триптофан и глюкоза. Глюкоза стимулирует повышенный выход инсулина в кровь, который даёт команду основным аминокислотам уйти из кровяного русла в депо, освобождая триптофану дорогу через гематоэнцефалический барьер в мозг на выработку серотонина.
Чтобы повысить уровень серотонина в плазме крови и, соответственно, в ЦНС, используются ингибиторы обратного захвата серотонина, например, сертралин. Эти препараты способны угнетать захват серотонина и тем самым повышать его концентрацию. Все лекарства этого ряда являются рецептурными препаратами и подлежат использованию только по назначению врача.
Патологии, связанные с серотонином[править | править код]
Дефицит или ингибирование серотонинергической передачи, например, вызванные снижением уровня серотонина в мозге является одним из факторов формирования депрессивных состояний, навязчивых расстройств и тяжелых форм мигрени.
Гиперактивация серотониновых рецепторов (например, при приёме некоторых наркотиков) может привести к галлюцинациям. C хронически повышенным уровнем их активности может быть связано развитие шизофрении[9].
Накопление серотонина в ЦНС вследствие приёма серотонинергических препаратов может приводить к возникновению серотонинового синдрома[10]:59.
Пищевые продукты[править | править код]
Пищевые продукты с повышенным содержанием триптофана (аминокислота, из которой образуется серотонин): молочные продукты (особенно сыр), финики, сливы, инжир, томаты[11], соя, чёрный шоколад, способствуют биосинтезу серотонина и часто улучшают настроение. Они же могут быть причиной острых токсических реакций (серотониновый синдром), если употребляются в больших количествах на фоне лечения некоторыми группами антидепрессантов — ингибиторами моноаминоксидазы (ИМАО) или селективными ингибиторами обратного захвата серотонина (СИОЗС).
См. также[править | править код]
- 5-Гидрокситриптофан
- Антидепрессанты
- MDMA
- ЛСД
Примечания[править | править код]
- ↑ Young SN (2007). “How to increase serotonin in the human brain without drugs”. Rev. Psychiatr. Neurosci. 32 (6): 394—99. PMC 2077351. PMID 18043762.
- ↑ Negri L (2006). “[Vittorio Erspamer (1909–1999)]”. Med Secoli [итал.]. 18 (1): 97—113. PMID 17526278.
- ↑ Rapport MM, Green AA, Page IH (1948). “Serum vasoconstrictor, serotonin; isolation and characterization”. J. Biol. Chem. 176 (3): 1243—51. PMID 18100415.
- ↑ B. M. Twarog and I. H. Page. Serotonin content of some mammaliantissues and urine and a method for its determination.Am J Physiol, 175(1):157-61, 1953.
- ↑ Гормон счастья: 95% серотонина находится в кишечнике
- ↑ Витамин D и Омега-3 жирные кислоты полезны при депрессии и психоневрологических заболеваниях
- ↑ Nelson DL (2004). “5-HT5 receptors”. Current drug targets. CNS and neurological disorders. 3 (1): 53—8. PMID 14965244.
- ↑ Isbister, G. K.; Bowe, S. J.; Dawson, A.; Whyte, I. M. (2004). “Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose”. J. Toxicol. Clin. Toxicol. 42 (3): 277—85. DOI:10.1081/CLT-120037428. PMID 15362595.
- ↑ Dysconnection in Schizophrenia: From Abnormal Synaptic Plasticity to Failures of Self-monitoring. Дата обращения 7 октября 2011. Архивировано 23 августа 2011 года.
- ↑ Волков В.П. Ятрогенные психонейросоматические синдромы. — Тверь: Триада, 2014. — 320 с.
- ↑ Д. Абсентис. Х&С. Шариков, эпифиз и серотонин.
Литература[править | править код]
- Ашмарин И. П., Ещенко Н. Д., Каразеева Е. П. Нейрохимия в таблицах и схемах. — М.: «Экзамен», 2007.
Ссылки[править | править код]
- Дубынин В. Серотонин. ИД «ПостНаука» (24 октября 2016). — лекция о превращениях триптофана, нейрохимии серотонина и действии антидепрессантов. Дата обращения 8 сентября 2017. Архивировано 8 сентября 2017 года.






















