-
Главная
- Правописание слова «костный»
Неверные варианты написания
косный
Верный вариант написания
костный
Данную непроизносимую согласную мы можем проверить подобрав проверочное слово кость, где мы отчетливо слышим букву т.
Похожие слова по данному правилу
А Б В Г Д Е Ж З И Й К Л М Н О П Р С Т У Ф Х Ц Ч Ш Щ Э Ю Я
до мо́зга косте́й
Рядом по алфавиту:
до мо́зга косте́й
домовни́чанье , -я
домовни́чать , -аю, -ает
домово́д , -а
домово́дка , -и, р. мн. -док
домово́дство , -а
домово́дческий
домово́й , -о́го
домо́вый
домога́ние , -я
домога́тельство , -а
домога́ться , -а́юсь, -а́ется
домоде́довский , (от Домоде́дово)
домоде́ланный
домоде́льный
домоде́льщина , -ы
домо́й
домо́к , домка́
домо́кнуть , -ну, -нет; прош. -о́к, -о́кла
домо́кший
домола́чивание , -я
домола́чивать(ся) , -аю, -ает(ся)
домолоти́ть(ся) , -очу́, -о́тит(ся)
домо́лотый
домоло́ть(ся) , домелю́, доме́лет(ся)
домоло́ченный , кр. ф. -ен, -ена
домонго́льский
домонополисти́ческий
домоправи́тель , -я
домоправи́тельница , -ы, тв. -ей
домоправле́ние , -я (занятие домоправителя)
Рады помочь вам узнать, как пишется слово «костно-мозговой».
Пишите и говорите правильно.
О словаре
Сайт создан на основе «Русского орфографического словаря», составленного Институтом русского языка имени В. В. Виноградова РАН. Объем второго издания, исправленного и дополненного, составляет около 180 тысяч слов, и существенно превосходит все предшествующие орфографические словари. Он является нормативным справочником, отражающим с возможной полнотой лексику русского языка начала 21 века и регламентирующим ее правописание.
Правильное написание слова испорченный до мозга костей:
испорченный до мозга костей
Крутая NFT игра. Играй и зарабатывай!
Правильный транслит слова: isporchenniy do mozga kostey
Написание с не правильной раскладкой клавиатуры: bcgjhxtyysq lj vjpuf rjcntq
Неправильное написание слова с ошибкой: испорченый до мозга костей
Тест на правописание
Энциклопедический словарь, 1998 г.
костный мозг
содержится во всех полостях костей у позвоночных животных и человека. В красном костном мозге, заполняющем в течение первых лет жизни все полости костей, образуются форменные элементы крови — эритроциты, лейкоциты и тромбоциты. Желтый костный мозг, замещающий постепенно красный, состоит главным образом из жировых клеток.
Большая Советская Энциклопедия
Костный мозг
ткань, заполняющая полости костей у позвоночных животных и человека. Различают красный К. м. с преобладанием кроветворной миелоидной ткани и жёлтый с преобладанием жировой ткани. Красный К. м. сохраняется в течение всей жизни в плоских костях (ребрах, грудине, костях черепа, таза), а также в позвонках и эпифазах трубчатых костей. У человека он составляет около 1,5% массы тела. С возрастом кроветворная ткань в полостях трубчатых костей замещается жировой и К. м. в них становится жёлтым.
Красный К. м. ≈ основной кроветворный орган у взрослых млекопитающих и человека. В нём происходит развитие эритроцитов, зернистых лейкоцитов (нейтрофилов, эозинофилов, базофилов), кровяных пластинок (тромбоцитов), а также костномозговых лимфоцитов. В состав К. м. (около 0,1% всех его клеток) входят особые, так называемые стволовые, кроветворные клетки. Стволовые клетки, благодаря их способности к многократному делению и развитию в направлении всех форм кроветворных и лимфоидных клеток, поддерживают кроветворение в К. м. и обеспечивают возмещение постоянно происходящей в организме убыли лейкоцитов и эритроцитов. Главную массу К. м. составляют созревающие клетки разных ростков кроветворения (эритроидных, миелоидных, лимфоцитов, мегакариоцитов). Все они ≈ потомки стволовых кроветворных клеток и пополняются за их счёт; часть из них способна к нескольким делениям. Относительное содержание в К. м. созревающих клеток отдельных ростков кроветворения и более или менее зрелых клеточных форм каждого из ростков служит важной характеристикой процесса кроветворения. По мере созревания клетки из К. м. поступают в кровяное русло. Кроме зрелых клеток, из К. м. выходит и некоторое количество стволовых кроветворных клеток, способных переселяться в др. кроветворные органы. Основу красного К. м. составляет ретикулярная ткань, образующая клеточный синцитий, на котором располагаются кроветворные клетки. Их размножение и созревание во многом зависят от взаимодействия с ретикулярной тканью, обладающей, кроме того, способностью к костеобразованию, что проявляется при заживлении переломов костей. Интенсивность кроветворения в К. м. может резко увеличиваться. Благодаря этому значительный убыль клеток крови (например, при кровопотерях) или разрушение значительной части клеток К. м. обычно быстро восполняются. Однако к некоторым воздействиям (например, ионизирующим излучениям ) К. м. и, в частности, его стволовые клетки высоко чувствительны. Поэтому состояние К. м. ≈ один из главных факторов, определяющих резистентность (устойчивость) организма к таким воздействиям.
Лит.: Заварзин А. А. и Румянцев А. В., Курс гистологии, 6 изд., М., 1946; Чертков И. Л. и Фриденштейн А. Я., Родоначальная кроветворная клетка и ее дифференцировка, «Успехи современной биологии», 1966, т. 62, в. 1.
А. Я. Фриденштейн.
Википедия
Костный мозг
Костный мозг — мягкая ткань внутренней полости кости. У людей в костном мозгу происходит гемопоэз . У человека костный мозг составляет в среднем 4 % массы тела.
Внутренние полости кости содержат мягкую, нежную, богатую клетками и снабжённую кровеносными сосудами массу, называемую костным мозгом (у птиц часть полостей наполнена воздухом). Различают три его вида: слизистый . Основную форму составляет красный костный мозг, в нём наблюдается нежная соединительно-тканная основа, богатая сосудами, очень похожие на лейкоциты костномозговые или лимфатические клетки, клетки, окрашенные гемоглобином и считаемые за переход к красным кровяным тельцам, бесцветные клетки, содержащие внутри красные шарики, и многоядерные крупные клетки, так называемые миэлопласты.
Красный (деятельный) костный мозг — это миелоидная ткань, которая, как и лимфоидная, состоит из двух основных компонентов: стромального — строма, служащая микроокружением для гемопоэтических клеток, и гемального — форменные элементы крови на разных стадиях развития.
Строма образована ретикулярной тканью, остеогенными, тучными, жировыми, адвентициальными, эндотелиальными клетками и межклеточным веществом.
Желтый (недеятельный) костный мозг — это жировая ткань с отдельными островками ретикулярной ткани. Он находится в костномозговых каналах трубчатых костей и в частях ячеек губчатого вещества костей.
Слизистый костный мозг — студенистая, слизистая, бедная клетками консистенция. Он образуется в развивающихся костях черепа.
При отложении в стромальный компонент основы жира и уменьшении числа миелоидных элементов красный мозг переходит в жёлтый, а при исчезновении жира и миелоидных элементов он приближается к слизистому.
Костный мозг не имеет ничего общего с головным и спинным мозгом. Он не относится к нервной системе и не имеет нейронов.
Как правильно пишется словосочетание «костный мозг»
- Как правильно пишется слово «костный»
- Как правильно пишется слово «мозг»
Делаем Карту слов лучше вместе
Привет! Меня зовут Лампобот, я компьютерная программа, которая помогает делать
Карту слов. Я отлично
умею считать, но пока плохо понимаю, как устроен ваш мир. Помоги мне разобраться!
Спасибо! Я стал чуточку лучше понимать мир эмоций.
Вопрос: цезий — это что-то нейтральное, положительное или отрицательное?
Ассоциации к словосочетанию «костный мозг»
Синонимы к словосочетанию «костный мозг»
Предложения со словосочетанием «костный мозг»
- Этот тип реакции наблюдается при трансплантациях, а также при пересадке костного мозга.
- Все они так или иначе столкнулись с гибелью клеток костного мозга.
- Ежедневное употребление 4–5 г сухой биомассы спирулины способствует полному восстановлению функций красного костного мозга в течение нескольких месяцев и очищает организм от остаточных радионуклидов.
- (все предложения)
Цитаты из русской классики со словосочетанием «костный мозг»
- Но не в этом дело. Я только прошу снизойти к моей слабости и понять, что оторвать от кафедры и учеников человека, которого судьбы костного мозга интересуют больше, чем конечная цель мироздания, равносильно тому, если бы его взяли да и заколотили в гроб, не дожидаясь, пока он умрет.
- (все
цитаты из русской классики)
Сочетаемость слова «мозг»
- головной мозг
человеческий мозг
спинной мозг - до мозга костей
мозг человека
мозг ребёнка - сотрясение мозга
кора головного мозга
работа мозга - мозг работает
мозг отключился
мозг понимает - пораскинуть мозгами
шевелить мозгами
проникнуть в мозг - (полная таблица сочетаемости)
Значение словосочетания «костный мозг»
-
Костный мозг — мягкая ткань внутренней полости кости. У людей в костном мозгу происходит гемопоэз. У человека костный мозг составляет в среднем 4 % массы тела. (Википедия)
Все значения словосочетания КОСТНЫЙ МОЗГ
Афоризмы русских писателей со словом «мозг»
- Со времени Аристотеля человеческий мозг не изменился. Тем более не изменилось человеческое сознание.
А значит, нет прогресса. Есть — движение, в основе которого лежит неустойчивость. - Вращаться в мире чудесных, глубоких загадок бытия, тратить энергию своего мозга на разрешение их — вот истинно человеческая жизнь, вот где неисчерпаемый источник счастья и животворной радости!
- Существует только человек, все же остальное — дело его рук и его мозга!
- (все афоризмы русских писателей)
Отправить комментарий
Дополнительно
Косный или костный
Слово «костный» с буквой Т имеет значение «связанный с костями». Например, костный мозг, костная мука. Слово «косный» пишется без буквы Т и имеет значение «что-то устоявшееся, патриархальное, отстающее от современной жизни». Например, косный человек, косный ум.
Косный или костный
| Bone marrow | |
|---|---|
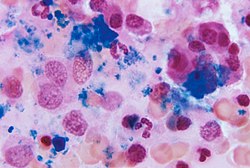
A section of bone marrow tissue |
|
| Details | |
| System | Hematopoietic system Immune system[1] Lymphatic system |
| Identifiers | |
| Latin | Medulla ossium |
| MeSH | D001853 |
| TA98 | A13.1.01.001 |
| TA2 | 388 |
| FMA | 9608 |
| Anatomical terminology
[edit on Wikidata] |
Bone marrow is a semi-solid tissue found within the spongy (also known as cancellous) portions of bones.[2] In birds and mammals, bone marrow is the primary site of new blood cell production (or haematopoiesis).[3] It is composed of hematopoietic cells,[4] marrow adipose tissue, and supportive stromal cells. In adult humans, bone marrow is primarily located in the ribs, vertebrae, sternum, and bones of the pelvis.[5] Bone marrow comprises approximately 5% of total body mass in healthy adult humans, such that a man weighing 73 kg (161 lbs) will have around 3.7 kg (8 lbs) of bone marrow.[6]
Human marrow produces approximately 500 billion blood cells per day, which join the systemic circulation via permeable vasculature sinusoids within the medullary cavity.[7] All types of hematopoietic cells, including both myeloid and lymphoid lineages, are created in bone marrow; however, lymphoid cells must migrate to other lymphoid organs (e.g. thymus) in order to complete maturation.
Bone marrow transplants can be conducted to treat severe diseases of the bone marrow, including certain forms of cancer such as leukemia. Several types of stem cells are related to bone marrow. Hematopoietic stem cells in the bone marrow can give rise to hematopoietic lineage cells, and mesenchymal stem cells, which can be isolated from the primary culture of bone marrow stroma, can give rise to bone, adipose, and cartilage tissue.[8]
Structure[edit]
The composition of marrow is dynamic, as the mixture of cellular and non-cellular components (connective tissue) shifts with age and in response to systemic factors. In humans, marrow is colloquially characterized as «red» or «yellow» marrow (Latin: medulla ossium rubra, Latin: medulla ossium flava, respectively) depending on the prevalence of hematopoietic cells vs fat cells. While the precise mechanisms underlying marrow regulation are not understood,[7] compositional changes occur according to stereotypical patterns.[9] For example, a newborn baby’s bones exclusively contain hematopoietically active «red» marrow, and there is a progressive conversion towards «yellow» marrow with age. In adults, red marrow is found mainly in the central skeleton, such as the pelvis, sternum, cranium, ribs, vertebrae and scapulae, and variably found in the proximal epiphyseal ends of long bones such as the femur and humerus. In circumstances of chronic hypoxia, the body can convert yellow marrow back to red marrow to increase blood cell production.[10]
Hematopoietic components[edit]
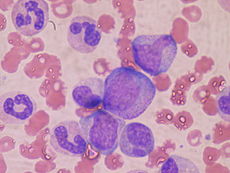
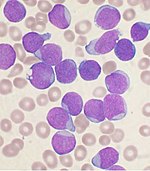
At the cellular level, the main functional component of bone marrow includes the progenitor cells which are destined to mature into blood and lymphoid cells. Human marrow produces approximately 500 billion blood cells per day.[11] Marrow contains hematopoietic stem cells which give rise to the three classes of blood cells that are found in circulation: white blood cells (leukocytes), red blood cells (erythrocytes), and platelets (thrombocytes).[12]
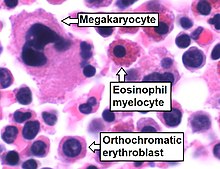
Cellular constitution of the red bone marrow parenchyma[13]
| Group | Cell type | Average fraction |
Reference range |
|---|---|---|---|
| Myelopoietic cells |
Myeloblasts | 0.9 | 0.2–1.5 |
| Promyelocytes | 3.3% | 2.1–4.1 | |
| Neutrophilic myelocytes | 12.7% | 8.2–15.7 | |
| Eosinophilic myelocytes | 0.8% | 0.2–1.3 | |
| Neutrophilic metamyelocytes | 15.9% | 9.6–24.6 | |
| Eosinophilic metamyelocytes | 1.2% | 0.4–2.2 | |
| Neutrophilic band cells | 12.4% | 9.5–15.3 | |
| Eosinophilic band cells | 0.9% | 0.2–2.4 | |
| Segmented neutrophils | 7.4% | 6.0–12.0 | |
| Segmented eosinophils | 0.5% | 0.0–1.3 | |
| Segmented basophils and mast cells | 0.1% | 0.0–0.2 | |
| Erythropoietic cells |
Pronormoblasts | 0.6% | 0.2–1.3 |
| Basophilic normoblasts | 1.4% | 0.5–2.4 | |
| Polychromatic normoblasts | 21.6% | 17.9–29.2 | |
| Orthochromatic normoblast | 2.0% | 0.4–4.6 | |
| Other cell types |
Megakaryocytes | < 0.1% | 0.0-0.4 |
| Plasma cells | 1.3% | 0.4-3.9 | |
| Reticular cells | 0.3% | 0.0-0.9 | |
| Lymphocytes | 16.2% | 11.1-23.2 | |
| Monocytes | 0.3% | 0.0-0.8 |
Stroma[edit]
The stroma of the bone marrow includes all tissue not directly involved in the marrow’s primary function of hematopoiesis.[7] Stromal cells may be indirectly involved in hematopoiesis, providing a microenvironment that influences the function and differentiation of hematopoietic cells. For instance, they generate colony stimulating factors, which have a significant effect on hematopoiesis. Cell types that constitute the bone marrow stroma include:
- fibroblasts (reticular connective tissue)
- macrophages, which contribute especially to red blood cell production, as they deliver iron for hemoglobin production.
- adipocytes (fat cells)
- osteoblasts (synthesize bone)
- osteoclasts (resorb bone)
- endothelial cells, which form the sinusoids. These derive from endothelial stem cells, which are also present in the bone marrow.[12]
Function[edit]
Mesenchymal stem cells[edit]
The bone marrow stroma contains mesenchymal stem cells (MSCs),[12] which are also known as marrow stromal cells. These are multipotent stem cells that can differentiate into a variety of cell types. MSCs have been shown to differentiate, in vitro or in vivo, into osteoblasts, chondrocytes, myocytes, marrow adipocytes and beta-pancreatic islets cells.[citation needed]
Bone marrow barrier[edit]
The blood vessels of the bone marrow constitute a barrier, inhibiting immature blood cells from leaving the marrow. Only mature blood cells contain the membrane proteins, such as aquaporin and glycophorin, that are required to attach to and pass the blood vessel endothelium.[14] Hematopoietic stem cells may also cross the bone marrow barrier, and may thus be harvested from blood.[citation needed]
Lymphatic role[edit]
The red bone marrow is a key element of the lymphatic system, being one of the primary lymphoid organs that generate lymphocytes from immature hematopoietic progenitor cells.[15] The bone marrow and thymus constitute the primary lymphoid tissues involved in the production and early selection of lymphocytes. Furthermore, bone marrow performs a valve-like function to prevent the backflow of lymphatic fluid in the lymphatic system.[citation needed]
Compartmentalization[edit]
Biological compartmentalization is evident within the bone marrow, in that certain cell types tend to aggregate in specific areas. For instance, erythrocytes, macrophages, and their precursors tend to gather around blood vessels, while granulocytes gather at the borders of the bone marrow.[12]
As food[edit]
People have used animal bone-marrow in cuisine worldwide for millennia, as in the famed Milanese Ossobuco.[16]
Marrow oil[edit]
An 1892 text provides the following instructions and comments on hair oil:
Marrow oil.-
- Clarified beef marrow …….. 1+1⁄2oz.
- Oil of almonds ……………….. 1⁄4 pt.
Melt them together, and scent the mixture at will. Held in high repute as a hair oil by many. That of the shops has seldom any marrow in it, but lard instead. The appropriate scents are the same as for bears’ grease. It is generally tinged slightly yellow by means of a little palm oil or annatto.[17]
Clinical significance[edit]
Disease[edit]
The normal bone marrow architecture can be damaged or displaced by aplastic anemia, malignancies such as multiple myeloma, or infections such as tuberculosis, leading to a decrease in the production of blood cells and blood platelets. The bone marrow can also be affected by various forms of leukemia, which attacks its hematologic progenitor cells.[18] Furthermore, exposure to radiation or chemotherapy will kill many of the rapidly dividing cells of the bone marrow, and will therefore result in a depressed immune system. Many of the symptoms of radiation poisoning are due to damage sustained by the bone marrow cells.[citation needed]
To diagnose diseases involving the bone marrow, a bone marrow aspiration is sometimes performed. This typically involves using a hollow needle to acquire a sample of red bone marrow from the crest of the ilium under general or local anesthesia.[19]
Application of stem cells in therapeutics[edit]
Bone marrow derived stem cells have a wide array of application in regenerative medicine.[20]
Imaging[edit]
Medical imaging may provide a limited amount of information regarding bone marrow. Plain film x-rays pass through soft tissues such as marrow and do not provide visualization, although any changes in the structure of the associated bone may be detected.[21] CT imaging has somewhat better capacity for assessing the marrow cavity of bones, although with low sensitivity and specificity. For example, normal fatty «yellow» marrow in adult long bones is of low density (-30 to -100 Hounsfield units), between subcutaneous fat and soft tissue. Tissue with increased cellular composition, such as normal «red» marrow or cancer cells within the medullary cavity will measure variably higher in density.[22]
MRI is more sensitive and specific for assessing bone composition. MRI enables assessment of the average molecular composition of soft tissues and thus provides information regarding the relative fat content of marrow. In adult humans, «yellow» fatty marrow is the dominant tissue in bones, particularly in the (peripheral) appendicular skeleton. Because fat molecules have a high T1-relaxivity, T1-weighted imaging sequences show «yellow» fatty marrow as bright (hyperintense). Furthermore, normal fatty marrow loses signal on fat-saturation sequences, in a similar pattern to subcutaneous fat.[citation needed]
When «yellow» fatty marrow becomes replaced by tissue with more cellular composition, this change is apparent as decreased brightness on T1-weighted sequences. Both normal «red» marrow and pathologic marrow lesions (such as cancer) are darker than «yellow» marrow on T1-weight sequences, although can often be distinguished by comparison with the MR signal intensity of adjacent soft tissues. Normal «red» marrow is typically equivalent or brighter than skeletal muscle or intervertebral disc on T1-weighted sequences.[9][23]
Fatty marrow change, the inverse of red marrow hyperplasia, can occur with normal aging,[24] though it can also be seen with certain treatments such as radiation therapy. Diffuse marrow T1 hypointensity without contrast enhancement or cortical discontinuity suggests red marrow conversion or myelofibrosis. Falsely normal marrow on T1 can be seen with diffuse multiple myeloma or leukemic infiltration when the water to fat ratio is not sufficiently altered, as may be seen with lower grade tumors or earlier in the disease process.[25]
Histology[edit]
Bone marrow examination is the pathologic analysis of samples of bone marrow obtained via biopsy and bone marrow aspiration. Bone marrow examination is used in the diagnosis of a number of conditions, including leukemia, multiple myeloma, anemia, and pancytopenia. The bone marrow produces the cellular elements of the blood, including platelets, red blood cells and white blood cells. While much information can be gleaned by testing the blood itself (drawn from a vein by phlebotomy), it is sometimes necessary to examine the source of the blood cells in the bone marrow to obtain more information on hematopoiesis; this is the role of bone marrow aspiration and biopsy.[citation needed]
The ratio between myeloid series and erythroid cells is relevant to bone marrow function, and also to diseases of the bone marrow and peripheral blood, such as leukemia and anemia. The normal myeloid-to-erythroid ratio is around 3:1; this ratio may increase in myelogenous leukemias, decrease in polycythemias, and reverse in cases of thalassemia.[26]
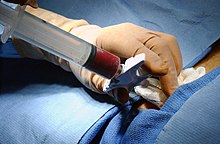
Donation and transplantation[edit]
A bone marrow harvest in progress.
The preferred sites for the procedure
In a bone marrow transplant, hematopoietic stem cells are removed from a person and infused into another person (allogenic) or into the same person at a later time (autologous). If the donor and recipient are compatible, these infused cells will then travel to the bone marrow and initiate blood cell production. Transplantation from one person to another is conducted for the treatment of severe bone marrow diseases, such as congenital defects, autoimmune diseases or malignancies. The patient’s own marrow is first killed off with drugs or radiation, and then the new stem cells are introduced. Before radiation therapy or chemotherapy in cases of cancer, some of the patient’s hematopoietic stem cells are sometimes harvested and later infused back when the therapy is finished to restore the immune system.[27]
Bone marrow stem cells can be induced to become neural cells to treat neurological illnesses,[28] and can also potentially be used for the treatment of other illnesses, such as inflammatory bowel disease.[29] In 2013, following a clinical trial, scientists proposed that bone marrow transplantation could be used to treat HIV in conjunction with antiretroviral drugs;[30][31] however, it was later found that HIV remained in the bodies of the test subjects.[32]
Harvesting[edit]
The stem cells are typically harvested directly from the red marrow in the iliac crest, often under general anesthesia. The procedure is minimally invasive and does not require stitches afterwards. Depending on the donor’s health and reaction to the procedure, the actual harvesting can be an outpatient procedure, or can require 1–2 days of recovery in the hospital.[33]
Another option is to administer certain drugs that stimulate the release of stem cells from the bone marrow into circulating blood.[34] An intravenous catheter is inserted into the donor’s arm, and the stem cells are then filtered out of the blood. This procedure is similar to that used in blood or platelet donation. In adults, bone marrow may also be taken from the sternum, while the tibia is often used when taking samples from infants.[19] In newborns, stem cells may be retrieved from the umbilical cord.[35]
Persistent viruses[edit]
Using quantitative Polymerase Chain Reaction (qPCR) and Next-generation Sequencing (NGS) a maximum of five DNA viruses per individual have been identified. Included were several herpesviruses, hepatitis B virus, Merkel cell polyomavirus, and human papillomavirus 31. Given the reactivation and/or oncogenic potential of these viruses, their repercussion on hematopoietic and malignant disorders calls for further studies.[36]
Fossil record[edit]
Bone marrow may have first evolved in Eusthenopteron, a species of prehistoric fish with close links to early tetrapods.
The earliest fossilised evidence of bone marrow was discovered in 2014 in Eusthenopteron, a lobe-finned fish which lived during the Devonian period approximately 370 million years ago.[37] Scientists from Uppsala University and the European Synchrotron Radiation Facility used X-ray synchrotron microtomography to study the fossilised interior of the skeleton’s humerus, finding organised tubular structures akin to modern vertebrate bone marrow.[37] Eusthenopteron is closely related to the early tetrapods, which ultimately evolved into the land-dwelling mammals and lizards of the present day.[37]
See also[edit]
- Myelonecrosis
- National Marrow Donor Program
- Gift of Life Marrow Registry
References[edit]
- ^ Schmidt, Richard F.; Lang, Florian; Heckmann, Manfred (30 November 2010). What are the organs of the immune system?. Institute for Quality and Efficiency in Health Care. pp. 3/7.
- ^ C., Farhi, Diane (2009). Pathology of bone marrow and blood cells (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott William & Wilkins. ISBN 9780781770934. OCLC 191807944.
- ^ Arikan, Hüseyin; Çiçek, Kerim (2014). «Haematology of amphibians and reptiles: a review» (PDF). North-Western Journal of Zoology. 10: 190–209.
- ^ Monga I, Kaur K, Dhanda S (March 2022). «Revisiting hematopoiesis: applications of the bulk and single-cell transcriptomics dissecting transcriptional heterogeneity in hematopoietic stem cells». Briefings in Functional Genomics. 21 (3): 159–176. doi:10.1093/bfgp/elac002. PMID 35265979.
- ^ Katherine, Abel (2013). Official CPC Certification Study Guide. American Medical Association.
- ^ Hindorf, C.; Glatting, G.; Chiesa, C.; Lindén, O.; Flux, G. (2010). «EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry». Eur J Nucl Med Mol Imaging. 37 (6): 1238–1250. doi:10.1007/s00259-010-1422-4. PMID 20411259. S2CID 9755621.
- ^ a b c Birbrair, Alexander; Frenette, Paul S. (1 March 2016). «Niche heterogeneity in the bone marrow». Annals of the New York Academy of Sciences. 1370 (1): 82–96. Bibcode:2016NYASA1370…82B. doi:10.1111/nyas.13016. ISSN 1749-6632. PMC 4938003. PMID 27015419.
- ^ Lindberg, Matthew R.; Lamps, Laura W. (2018). «Bone Marrow». Diagnostic Pathology: Normal Histology. pp. 130–137. doi:10.1016/B978-0-323-54803-8.50035-8. ISBN 9780323548038.
- ^ a b Chan, Brian Y.; Gill, Kara G.; Rebsamen, Susan L.; Nguyen, Jie C. (1 October 2016). «MR Imaging of Pediatric Bone Marrow». RadioGraphics. 36 (6): 1911–1930. doi:10.1148/rg.2016160056. ISSN 0271-5333. PMID 27726743.
- ^ Poulton, T B; Murphy, W D; Duerk, J L; Chapek, C C; Feiglin, D H (1 December 1993). «Bone marrow reconversion in adults who are smokers: MR Imaging findings». American Journal of Roentgenology. 161 (6): 1217–1221. doi:10.2214/ajr.161.6.8249729. ISSN 0361-803X. PMID 8249729.
- ^ Nombela-Arrieta, Cesar; G. Manz, Markus (2017). «Quantification and three-dimensional microanatomical organization of the bone marrow». Blood Advances. 1 (6): 407–416. doi:10.1182/bloodadvances.2016003194. PMC 5738992. PMID 29296956.
- ^ a b c d Raphael Rubin & David S. Strayer (2007). Rubin’s Pathology: Clinicopathologic Foundations of Medicine. Lippincott Williams & Wilkins. p. 90. ISBN 978-0-7817-9516-6.
- ^ Appendix A:IV in Wintrobe’s clinical hematology (9th edition). Philadelphia: Lea & Febiger (1993).
- ^ «The Red Cell Membrane: structure and pathologies» (PDF). Australian Centre for Blood Diseases/Monash University. Retrieved 24 January 2015.
- ^ The Lymphatic System. Allonhealth.com. Retrieved 5 December 2011.
- ^
Fabricant, Florence. «Begging for Bones: A New Craving for Marrow». The New York Times. September 16, 1998. - ^
Hopkins, Albert Allis, ed. (1892). «Hair». The Scientific American Cyclopedia of Receipts, Notes and Queries. New York: Munn & Company. p. 255. Retrieved 18 December 2022. - ^ Bonnet, D; Dick, JE (1997). «Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell». Nature Medicine. 3 (7): 730–737. doi:10.1038/nm0797-730. PMID 9212098. S2CID 205381050.
- ^ a b «Bone Marrow Aspiration and Biopsy». Lab Tests Online UK. Retrieved 16 February 2013.
- ^
Mahla RS (2016). «Stem cells application in regenerative medicine and disease threpeutics». International Journal of Cell Biology. 2016 (7): 1–24. doi:10.1155/2016/6940283. PMC 4969512. PMID 27516776.{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ Ellmann, Stephan; Beck, Michael; Kuwert, Torsten; Uder, Michael; Bäuerle, Tobias (2015). «Multimodal imaging of bone metastases: From preclinical to clinical applications». Journal of Orthopaedic Translation. 3 (4): 166–177. doi:10.1016/j.jot.2015.07.004. PMC 5986987. PMID 30035055.
- ^ Nishida, Y; Matsue, Y; Suehara, Y; Fukumoto, K; Fujisawa, M; Takeuchi, M; Ouchi, E; Matsue, K (August 2015). «Clinical and prognostic significance of bone marrow abnormalities in the appendicular skeleton detected by low-doe whole-body multidetector computed tomography in patients with multiple myeloma». Blood Cancer Journal. 5 (7): e329. doi:10.1038/bcj.2015.57. ISSN 2044-5385. PMC 4526783. PMID 26230953.
- ^ Poulton, TB; Murphy, WD; Duerk, JL; Chapek, CC; Feiglin, DH (December 1993). «Bone marrow reconversion in adults who are smokers: MR Imaging findings». AJR. American Journal of Roentgenology. 161 (6): 1217–21. doi:10.2214/ajr.161.6.8249729. PMID 8249729.
- ^ Shah, LM; Hanrahan, CJ (December 2011). «MRI of spinal bone marrow: part I, techniques and normal age-related appearances». AJR. American Journal of Roentgenology. 197 (6): 1298–308. doi:10.2214/ajr.11.7005. PMID 22109283. S2CID 20115888.
- ^ Vande Berg, BC; Lecouvet, FE; Galant, C; Maldague, BE; Malghem, J (July 2005). «Normal variants and frequent marrow alterations that simulate bone marrow lesions at MR imaging». Radiologic Clinics of North America. 43 (4): 761–70, ix. doi:10.1016/j.rcl.2005.01.007. PMID 15893536.
- ^ «Definition: ‘M:E Ratio’«. Stedman’s Medical Dictionary via MediLexicon.com. 2006. Archived from the original on 10 May 2013. Retrieved 20 December 2012.
- ^ «Bone marrow transplantation». UpToDate.com. Retrieved 12 April 2014.
- ^ «Antibody Transforms Stem Cells Directly Into Brain Cells». Science Daily. 22 April 2013. Retrieved 24 April 2013.
- ^ «Research Supports Promise of Cell Therapy for Bowel Disease». Wake Forest Baptist Medical Center. 28 February 2013. Retrieved 5 March 2013.
- ^ «Bone marrow ‘frees men of HIV drugs’«. BBC. 3 July 2013. Retrieved 3 July 2013.
- ^ «Stem-Cell Transplants Erase HIV In Two Men». PopSci. 3 July 2013. Retrieved 3 July 2013.
- ^ «HIV Returns in Two Men Thought ‘Cured’ by Bone Marrow Transplants». RH Reality Check. 10 December 2013. Retrieved 10 December 2013.
- ^ National Marrow Donor Program Donor Guide Archived 8 September 2008 at the Wayback Machine. Marrow.org. Retrieved 5 November 2012.
- ^ Bone marrow donation: What to expect when you donate. Mayo Clinic. Retrieved 16 February 2013.
- ^ McGuckin, C. P.; Forraz, N.; Baradez, M. -O.; Navran, S.; Zhao, J.; Urban, R.; Tilton, R.; Denner, L. (2005). «Production of stem cells with embryonic characteristics from human umbilical cord blood». Cell Proliferation. 38 (4): 245–255. doi:10.1111/j.1365-2184.2005.00346.x. PMC 6496335. PMID 16098183.
- ^ Toppinen, Mari; Sajantila, Antti; Pratas, Diogo; Hedman, Klaus; Perdomo, Maria F. (2021). «The Human Bone Marrow Is Host to the DNAs of Several Viruses». Front. Cell. Infect. Microbiol. 11: 657245. doi:10.3389/fcimb.2021.657245. PMC 8100435. PMID 33968803.
- ^ a b c Sanchez, S.; Tafforeau, P.; Ahlberg, P. E. (2014). «The humerus of Eusthenopteron: a puzzling organization presaging the establishment of tetrapod limb bone marrow». Proceedings of the Royal Society B: Biological Sciences. 281 (1782): 20140299. doi:10.1098/rspb.2014.0299. PMC 3973280. PMID 24648231.
Further reading[edit]
- Nature Bone Marrow Transplantation (Nature Publishing Group) – specialist scientific journal with articles on bone marrow biology and clinical uses.
- Cooper, B (2011). «The origins of bone marrow as the seedbed of our blood: from antiquity to the time of Osler». Baylor University Medical Center Proceedings. 24 (2): 115–8. doi:10.1080/08998280.2011.11928697. PMC 3069519. PMID 21566758.
- Wang J, Liu X, Lu H, Jiang C, Cui X, Yu L, Fu X, Li Q, Wang J (2015). «CXCR4(+)CD45(-) BMMNC subpopulation is superior to unfractionated BMMNCs for protection after ischemic stroke in mice». Brain Behav. Immun. 45: 98–108. doi:10.1016/j.bbi.2014.12.015. PMC 4342301. PMID 25526817.
External links[edit]
- Bone marrow histology photomicrographs
| Bone marrow | |
|---|---|

A section of bone marrow tissue |
|
| Details | |
| System | Hematopoietic system Immune system[1] Lymphatic system |
| Identifiers | |
| Latin | Medulla ossium |
| MeSH | D001853 |
| TA98 | A13.1.01.001 |
| TA2 | 388 |
| FMA | 9608 |
| Anatomical terminology
[edit on Wikidata] |
Bone marrow is a semi-solid tissue found within the spongy (also known as cancellous) portions of bones.[2] In birds and mammals, bone marrow is the primary site of new blood cell production (or haematopoiesis).[3] It is composed of hematopoietic cells,[4] marrow adipose tissue, and supportive stromal cells. In adult humans, bone marrow is primarily located in the ribs, vertebrae, sternum, and bones of the pelvis.[5] Bone marrow comprises approximately 5% of total body mass in healthy adult humans, such that a man weighing 73 kg (161 lbs) will have around 3.7 kg (8 lbs) of bone marrow.[6]
Human marrow produces approximately 500 billion blood cells per day, which join the systemic circulation via permeable vasculature sinusoids within the medullary cavity.[7] All types of hematopoietic cells, including both myeloid and lymphoid lineages, are created in bone marrow; however, lymphoid cells must migrate to other lymphoid organs (e.g. thymus) in order to complete maturation.
Bone marrow transplants can be conducted to treat severe diseases of the bone marrow, including certain forms of cancer such as leukemia. Several types of stem cells are related to bone marrow. Hematopoietic stem cells in the bone marrow can give rise to hematopoietic lineage cells, and mesenchymal stem cells, which can be isolated from the primary culture of bone marrow stroma, can give rise to bone, adipose, and cartilage tissue.[8]
Structure[edit]
The composition of marrow is dynamic, as the mixture of cellular and non-cellular components (connective tissue) shifts with age and in response to systemic factors. In humans, marrow is colloquially characterized as «red» or «yellow» marrow (Latin: medulla ossium rubra, Latin: medulla ossium flava, respectively) depending on the prevalence of hematopoietic cells vs fat cells. While the precise mechanisms underlying marrow regulation are not understood,[7] compositional changes occur according to stereotypical patterns.[9] For example, a newborn baby’s bones exclusively contain hematopoietically active «red» marrow, and there is a progressive conversion towards «yellow» marrow with age. In adults, red marrow is found mainly in the central skeleton, such as the pelvis, sternum, cranium, ribs, vertebrae and scapulae, and variably found in the proximal epiphyseal ends of long bones such as the femur and humerus. In circumstances of chronic hypoxia, the body can convert yellow marrow back to red marrow to increase blood cell production.[10]
Hematopoietic components[edit]
At the cellular level, the main functional component of bone marrow includes the progenitor cells which are destined to mature into blood and lymphoid cells. Human marrow produces approximately 500 billion blood cells per day.[11] Marrow contains hematopoietic stem cells which give rise to the three classes of blood cells that are found in circulation: white blood cells (leukocytes), red blood cells (erythrocytes), and platelets (thrombocytes).[12]
Cellular constitution of the red bone marrow parenchyma[13]
| Group | Cell type | Average fraction |
Reference range |
|---|---|---|---|
| Myelopoietic cells |
Myeloblasts | 0.9 | 0.2–1.5 |
| Promyelocytes | 3.3% | 2.1–4.1 | |
| Neutrophilic myelocytes | 12.7% | 8.2–15.7 | |
| Eosinophilic myelocytes | 0.8% | 0.2–1.3 | |
| Neutrophilic metamyelocytes | 15.9% | 9.6–24.6 | |
| Eosinophilic metamyelocytes | 1.2% | 0.4–2.2 | |
| Neutrophilic band cells | 12.4% | 9.5–15.3 | |
| Eosinophilic band cells | 0.9% | 0.2–2.4 | |
| Segmented neutrophils | 7.4% | 6.0–12.0 | |
| Segmented eosinophils | 0.5% | 0.0–1.3 | |
| Segmented basophils and mast cells | 0.1% | 0.0–0.2 | |
| Erythropoietic cells |
Pronormoblasts | 0.6% | 0.2–1.3 |
| Basophilic normoblasts | 1.4% | 0.5–2.4 | |
| Polychromatic normoblasts | 21.6% | 17.9–29.2 | |
| Orthochromatic normoblast | 2.0% | 0.4–4.6 | |
| Other cell types |
Megakaryocytes | < 0.1% | 0.0-0.4 |
| Plasma cells | 1.3% | 0.4-3.9 | |
| Reticular cells | 0.3% | 0.0-0.9 | |
| Lymphocytes | 16.2% | 11.1-23.2 | |
| Monocytes | 0.3% | 0.0-0.8 |
Stroma[edit]
The stroma of the bone marrow includes all tissue not directly involved in the marrow’s primary function of hematopoiesis.[7] Stromal cells may be indirectly involved in hematopoiesis, providing a microenvironment that influences the function and differentiation of hematopoietic cells. For instance, they generate colony stimulating factors, which have a significant effect on hematopoiesis. Cell types that constitute the bone marrow stroma include:
- fibroblasts (reticular connective tissue)
- macrophages, which contribute especially to red blood cell production, as they deliver iron for hemoglobin production.
- adipocytes (fat cells)
- osteoblasts (synthesize bone)
- osteoclasts (resorb bone)
- endothelial cells, which form the sinusoids. These derive from endothelial stem cells, which are also present in the bone marrow.[12]
Function[edit]
Mesenchymal stem cells[edit]
The bone marrow stroma contains mesenchymal stem cells (MSCs),[12] which are also known as marrow stromal cells. These are multipotent stem cells that can differentiate into a variety of cell types. MSCs have been shown to differentiate, in vitro or in vivo, into osteoblasts, chondrocytes, myocytes, marrow adipocytes and beta-pancreatic islets cells.[citation needed]
Bone marrow barrier[edit]
The blood vessels of the bone marrow constitute a barrier, inhibiting immature blood cells from leaving the marrow. Only mature blood cells contain the membrane proteins, such as aquaporin and glycophorin, that are required to attach to and pass the blood vessel endothelium.[14] Hematopoietic stem cells may also cross the bone marrow barrier, and may thus be harvested from blood.[citation needed]
Lymphatic role[edit]
The red bone marrow is a key element of the lymphatic system, being one of the primary lymphoid organs that generate lymphocytes from immature hematopoietic progenitor cells.[15] The bone marrow and thymus constitute the primary lymphoid tissues involved in the production and early selection of lymphocytes. Furthermore, bone marrow performs a valve-like function to prevent the backflow of lymphatic fluid in the lymphatic system.[citation needed]
Compartmentalization[edit]
Biological compartmentalization is evident within the bone marrow, in that certain cell types tend to aggregate in specific areas. For instance, erythrocytes, macrophages, and their precursors tend to gather around blood vessels, while granulocytes gather at the borders of the bone marrow.[12]
As food[edit]
People have used animal bone-marrow in cuisine worldwide for millennia, as in the famed Milanese Ossobuco.[16]
Marrow oil[edit]
An 1892 text provides the following instructions and comments on hair oil:
Marrow oil.-
- Clarified beef marrow …….. 1+1⁄2oz.
- Oil of almonds ……………….. 1⁄4 pt.
Melt them together, and scent the mixture at will. Held in high repute as a hair oil by many. That of the shops has seldom any marrow in it, but lard instead. The appropriate scents are the same as for bears’ grease. It is generally tinged slightly yellow by means of a little palm oil or annatto.[17]
Clinical significance[edit]
Disease[edit]
The normal bone marrow architecture can be damaged or displaced by aplastic anemia, malignancies such as multiple myeloma, or infections such as tuberculosis, leading to a decrease in the production of blood cells and blood platelets. The bone marrow can also be affected by various forms of leukemia, which attacks its hematologic progenitor cells.[18] Furthermore, exposure to radiation or chemotherapy will kill many of the rapidly dividing cells of the bone marrow, and will therefore result in a depressed immune system. Many of the symptoms of radiation poisoning are due to damage sustained by the bone marrow cells.[citation needed]
To diagnose diseases involving the bone marrow, a bone marrow aspiration is sometimes performed. This typically involves using a hollow needle to acquire a sample of red bone marrow from the crest of the ilium under general or local anesthesia.[19]
Application of stem cells in therapeutics[edit]
Bone marrow derived stem cells have a wide array of application in regenerative medicine.[20]
Imaging[edit]
Medical imaging may provide a limited amount of information regarding bone marrow. Plain film x-rays pass through soft tissues such as marrow and do not provide visualization, although any changes in the structure of the associated bone may be detected.[21] CT imaging has somewhat better capacity for assessing the marrow cavity of bones, although with low sensitivity and specificity. For example, normal fatty «yellow» marrow in adult long bones is of low density (-30 to -100 Hounsfield units), between subcutaneous fat and soft tissue. Tissue with increased cellular composition, such as normal «red» marrow or cancer cells within the medullary cavity will measure variably higher in density.[22]
MRI is more sensitive and specific for assessing bone composition. MRI enables assessment of the average molecular composition of soft tissues and thus provides information regarding the relative fat content of marrow. In adult humans, «yellow» fatty marrow is the dominant tissue in bones, particularly in the (peripheral) appendicular skeleton. Because fat molecules have a high T1-relaxivity, T1-weighted imaging sequences show «yellow» fatty marrow as bright (hyperintense). Furthermore, normal fatty marrow loses signal on fat-saturation sequences, in a similar pattern to subcutaneous fat.[citation needed]
When «yellow» fatty marrow becomes replaced by tissue with more cellular composition, this change is apparent as decreased brightness on T1-weighted sequences. Both normal «red» marrow and pathologic marrow lesions (such as cancer) are darker than «yellow» marrow on T1-weight sequences, although can often be distinguished by comparison with the MR signal intensity of adjacent soft tissues. Normal «red» marrow is typically equivalent or brighter than skeletal muscle or intervertebral disc on T1-weighted sequences.[9][23]
Fatty marrow change, the inverse of red marrow hyperplasia, can occur with normal aging,[24] though it can also be seen with certain treatments such as radiation therapy. Diffuse marrow T1 hypointensity without contrast enhancement or cortical discontinuity suggests red marrow conversion or myelofibrosis. Falsely normal marrow on T1 can be seen with diffuse multiple myeloma or leukemic infiltration when the water to fat ratio is not sufficiently altered, as may be seen with lower grade tumors or earlier in the disease process.[25]
Histology[edit]
Bone marrow examination is the pathologic analysis of samples of bone marrow obtained via biopsy and bone marrow aspiration. Bone marrow examination is used in the diagnosis of a number of conditions, including leukemia, multiple myeloma, anemia, and pancytopenia. The bone marrow produces the cellular elements of the blood, including platelets, red blood cells and white blood cells. While much information can be gleaned by testing the blood itself (drawn from a vein by phlebotomy), it is sometimes necessary to examine the source of the blood cells in the bone marrow to obtain more information on hematopoiesis; this is the role of bone marrow aspiration and biopsy.[citation needed]
The ratio between myeloid series and erythroid cells is relevant to bone marrow function, and also to diseases of the bone marrow and peripheral blood, such as leukemia and anemia. The normal myeloid-to-erythroid ratio is around 3:1; this ratio may increase in myelogenous leukemias, decrease in polycythemias, and reverse in cases of thalassemia.[26]
Donation and transplantation[edit]
A bone marrow harvest in progress.
The preferred sites for the procedure
In a bone marrow transplant, hematopoietic stem cells are removed from a person and infused into another person (allogenic) or into the same person at a later time (autologous). If the donor and recipient are compatible, these infused cells will then travel to the bone marrow and initiate blood cell production. Transplantation from one person to another is conducted for the treatment of severe bone marrow diseases, such as congenital defects, autoimmune diseases or malignancies. The patient’s own marrow is first killed off with drugs or radiation, and then the new stem cells are introduced. Before radiation therapy or chemotherapy in cases of cancer, some of the patient’s hematopoietic stem cells are sometimes harvested and later infused back when the therapy is finished to restore the immune system.[27]
Bone marrow stem cells can be induced to become neural cells to treat neurological illnesses,[28] and can also potentially be used for the treatment of other illnesses, such as inflammatory bowel disease.[29] In 2013, following a clinical trial, scientists proposed that bone marrow transplantation could be used to treat HIV in conjunction with antiretroviral drugs;[30][31] however, it was later found that HIV remained in the bodies of the test subjects.[32]
Harvesting[edit]
The stem cells are typically harvested directly from the red marrow in the iliac crest, often under general anesthesia. The procedure is minimally invasive and does not require stitches afterwards. Depending on the donor’s health and reaction to the procedure, the actual harvesting can be an outpatient procedure, or can require 1–2 days of recovery in the hospital.[33]
Another option is to administer certain drugs that stimulate the release of stem cells from the bone marrow into circulating blood.[34] An intravenous catheter is inserted into the donor’s arm, and the stem cells are then filtered out of the blood. This procedure is similar to that used in blood or platelet donation. In adults, bone marrow may also be taken from the sternum, while the tibia is often used when taking samples from infants.[19] In newborns, stem cells may be retrieved from the umbilical cord.[35]
Persistent viruses[edit]
Using quantitative Polymerase Chain Reaction (qPCR) and Next-generation Sequencing (NGS) a maximum of five DNA viruses per individual have been identified. Included were several herpesviruses, hepatitis B virus, Merkel cell polyomavirus, and human papillomavirus 31. Given the reactivation and/or oncogenic potential of these viruses, their repercussion on hematopoietic and malignant disorders calls for further studies.[36]
Fossil record[edit]
Bone marrow may have first evolved in Eusthenopteron, a species of prehistoric fish with close links to early tetrapods.
The earliest fossilised evidence of bone marrow was discovered in 2014 in Eusthenopteron, a lobe-finned fish which lived during the Devonian period approximately 370 million years ago.[37] Scientists from Uppsala University and the European Synchrotron Radiation Facility used X-ray synchrotron microtomography to study the fossilised interior of the skeleton’s humerus, finding organised tubular structures akin to modern vertebrate bone marrow.[37] Eusthenopteron is closely related to the early tetrapods, which ultimately evolved into the land-dwelling mammals and lizards of the present day.[37]
See also[edit]
- Myelonecrosis
- National Marrow Donor Program
- Gift of Life Marrow Registry
References[edit]
- ^ Schmidt, Richard F.; Lang, Florian; Heckmann, Manfred (30 November 2010). What are the organs of the immune system?. Institute for Quality and Efficiency in Health Care. pp. 3/7.
- ^ C., Farhi, Diane (2009). Pathology of bone marrow and blood cells (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott William & Wilkins. ISBN 9780781770934. OCLC 191807944.
- ^ Arikan, Hüseyin; Çiçek, Kerim (2014). «Haematology of amphibians and reptiles: a review» (PDF). North-Western Journal of Zoology. 10: 190–209.
- ^ Monga I, Kaur K, Dhanda S (March 2022). «Revisiting hematopoiesis: applications of the bulk and single-cell transcriptomics dissecting transcriptional heterogeneity in hematopoietic stem cells». Briefings in Functional Genomics. 21 (3): 159–176. doi:10.1093/bfgp/elac002. PMID 35265979.
- ^ Katherine, Abel (2013). Official CPC Certification Study Guide. American Medical Association.
- ^ Hindorf, C.; Glatting, G.; Chiesa, C.; Lindén, O.; Flux, G. (2010). «EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry». Eur J Nucl Med Mol Imaging. 37 (6): 1238–1250. doi:10.1007/s00259-010-1422-4. PMID 20411259. S2CID 9755621.
- ^ a b c Birbrair, Alexander; Frenette, Paul S. (1 March 2016). «Niche heterogeneity in the bone marrow». Annals of the New York Academy of Sciences. 1370 (1): 82–96. Bibcode:2016NYASA1370…82B. doi:10.1111/nyas.13016. ISSN 1749-6632. PMC 4938003. PMID 27015419.
- ^ Lindberg, Matthew R.; Lamps, Laura W. (2018). «Bone Marrow». Diagnostic Pathology: Normal Histology. pp. 130–137. doi:10.1016/B978-0-323-54803-8.50035-8. ISBN 9780323548038.
- ^ a b Chan, Brian Y.; Gill, Kara G.; Rebsamen, Susan L.; Nguyen, Jie C. (1 October 2016). «MR Imaging of Pediatric Bone Marrow». RadioGraphics. 36 (6): 1911–1930. doi:10.1148/rg.2016160056. ISSN 0271-5333. PMID 27726743.
- ^ Poulton, T B; Murphy, W D; Duerk, J L; Chapek, C C; Feiglin, D H (1 December 1993). «Bone marrow reconversion in adults who are smokers: MR Imaging findings». American Journal of Roentgenology. 161 (6): 1217–1221. doi:10.2214/ajr.161.6.8249729. ISSN 0361-803X. PMID 8249729.
- ^ Nombela-Arrieta, Cesar; G. Manz, Markus (2017). «Quantification and three-dimensional microanatomical organization of the bone marrow». Blood Advances. 1 (6): 407–416. doi:10.1182/bloodadvances.2016003194. PMC 5738992. PMID 29296956.
- ^ a b c d Raphael Rubin & David S. Strayer (2007). Rubin’s Pathology: Clinicopathologic Foundations of Medicine. Lippincott Williams & Wilkins. p. 90. ISBN 978-0-7817-9516-6.
- ^ Appendix A:IV in Wintrobe’s clinical hematology (9th edition). Philadelphia: Lea & Febiger (1993).
- ^ «The Red Cell Membrane: structure and pathologies» (PDF). Australian Centre for Blood Diseases/Monash University. Retrieved 24 January 2015.
- ^ The Lymphatic System. Allonhealth.com. Retrieved 5 December 2011.
- ^
Fabricant, Florence. «Begging for Bones: A New Craving for Marrow». The New York Times. September 16, 1998. - ^
Hopkins, Albert Allis, ed. (1892). «Hair». The Scientific American Cyclopedia of Receipts, Notes and Queries. New York: Munn & Company. p. 255. Retrieved 18 December 2022. - ^ Bonnet, D; Dick, JE (1997). «Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell». Nature Medicine. 3 (7): 730–737. doi:10.1038/nm0797-730. PMID 9212098. S2CID 205381050.
- ^ a b «Bone Marrow Aspiration and Biopsy». Lab Tests Online UK. Retrieved 16 February 2013.
- ^
Mahla RS (2016). «Stem cells application in regenerative medicine and disease threpeutics». International Journal of Cell Biology. 2016 (7): 1–24. doi:10.1155/2016/6940283. PMC 4969512. PMID 27516776.{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ Ellmann, Stephan; Beck, Michael; Kuwert, Torsten; Uder, Michael; Bäuerle, Tobias (2015). «Multimodal imaging of bone metastases: From preclinical to clinical applications». Journal of Orthopaedic Translation. 3 (4): 166–177. doi:10.1016/j.jot.2015.07.004. PMC 5986987. PMID 30035055.
- ^ Nishida, Y; Matsue, Y; Suehara, Y; Fukumoto, K; Fujisawa, M; Takeuchi, M; Ouchi, E; Matsue, K (August 2015). «Clinical and prognostic significance of bone marrow abnormalities in the appendicular skeleton detected by low-doe whole-body multidetector computed tomography in patients with multiple myeloma». Blood Cancer Journal. 5 (7): e329. doi:10.1038/bcj.2015.57. ISSN 2044-5385. PMC 4526783. PMID 26230953.
- ^ Poulton, TB; Murphy, WD; Duerk, JL; Chapek, CC; Feiglin, DH (December 1993). «Bone marrow reconversion in adults who are smokers: MR Imaging findings». AJR. American Journal of Roentgenology. 161 (6): 1217–21. doi:10.2214/ajr.161.6.8249729. PMID 8249729.
- ^ Shah, LM; Hanrahan, CJ (December 2011). «MRI of spinal bone marrow: part I, techniques and normal age-related appearances». AJR. American Journal of Roentgenology. 197 (6): 1298–308. doi:10.2214/ajr.11.7005. PMID 22109283. S2CID 20115888.
- ^ Vande Berg, BC; Lecouvet, FE; Galant, C; Maldague, BE; Malghem, J (July 2005). «Normal variants and frequent marrow alterations that simulate bone marrow lesions at MR imaging». Radiologic Clinics of North America. 43 (4): 761–70, ix. doi:10.1016/j.rcl.2005.01.007. PMID 15893536.
- ^ «Definition: ‘M:E Ratio’«. Stedman’s Medical Dictionary via MediLexicon.com. 2006. Archived from the original on 10 May 2013. Retrieved 20 December 2012.
- ^ «Bone marrow transplantation». UpToDate.com. Retrieved 12 April 2014.
- ^ «Antibody Transforms Stem Cells Directly Into Brain Cells». Science Daily. 22 April 2013. Retrieved 24 April 2013.
- ^ «Research Supports Promise of Cell Therapy for Bowel Disease». Wake Forest Baptist Medical Center. 28 February 2013. Retrieved 5 March 2013.
- ^ «Bone marrow ‘frees men of HIV drugs’«. BBC. 3 July 2013. Retrieved 3 July 2013.
- ^ «Stem-Cell Transplants Erase HIV In Two Men». PopSci. 3 July 2013. Retrieved 3 July 2013.
- ^ «HIV Returns in Two Men Thought ‘Cured’ by Bone Marrow Transplants». RH Reality Check. 10 December 2013. Retrieved 10 December 2013.
- ^ National Marrow Donor Program Donor Guide Archived 8 September 2008 at the Wayback Machine. Marrow.org. Retrieved 5 November 2012.
- ^ Bone marrow donation: What to expect when you donate. Mayo Clinic. Retrieved 16 February 2013.
- ^ McGuckin, C. P.; Forraz, N.; Baradez, M. -O.; Navran, S.; Zhao, J.; Urban, R.; Tilton, R.; Denner, L. (2005). «Production of stem cells with embryonic characteristics from human umbilical cord blood». Cell Proliferation. 38 (4): 245–255. doi:10.1111/j.1365-2184.2005.00346.x. PMC 6496335. PMID 16098183.
- ^ Toppinen, Mari; Sajantila, Antti; Pratas, Diogo; Hedman, Klaus; Perdomo, Maria F. (2021). «The Human Bone Marrow Is Host to the DNAs of Several Viruses». Front. Cell. Infect. Microbiol. 11: 657245. doi:10.3389/fcimb.2021.657245. PMC 8100435. PMID 33968803.
- ^ a b c Sanchez, S.; Tafforeau, P.; Ahlberg, P. E. (2014). «The humerus of Eusthenopteron: a puzzling organization presaging the establishment of tetrapod limb bone marrow». Proceedings of the Royal Society B: Biological Sciences. 281 (1782): 20140299. doi:10.1098/rspb.2014.0299. PMC 3973280. PMID 24648231.
Further reading[edit]
- Nature Bone Marrow Transplantation (Nature Publishing Group) – specialist scientific journal with articles on bone marrow biology and clinical uses.
- Cooper, B (2011). «The origins of bone marrow as the seedbed of our blood: from antiquity to the time of Osler». Baylor University Medical Center Proceedings. 24 (2): 115–8. doi:10.1080/08998280.2011.11928697. PMC 3069519. PMID 21566758.
- Wang J, Liu X, Lu H, Jiang C, Cui X, Yu L, Fu X, Li Q, Wang J (2015). «CXCR4(+)CD45(-) BMMNC subpopulation is superior to unfractionated BMMNCs for protection after ischemic stroke in mice». Brain Behav. Immun. 45: 98–108. doi:10.1016/j.bbi.2014.12.015. PMC 4342301. PMID 25526817.
External links[edit]
- Bone marrow histology photomicrographs
Русский[править]
Тип и синтаксические свойства сочетания[править]
ко́ст—ный мозг
Устойчивое сочетание (термин). Используется в качестве именной группы.
Произношение[править]
- МФА: [ˈkosnɨɪ̯ mosk]
Семантические свойства[править]
Значение[править]
- анат. мягкая сосудистая ткань, заполняющая полости костей позвоночных животных и человека и выполняющая функции гемопоэза (кроветворения) ◆ У млекопитающих эритроциты и лейкоциты формируются в костном мозге. Азимджан Турдыев, «Секрет Тортиллы, или радиация и кровь», 1975 г. // «Техника — молодёжи» [НКРЯ]
Синонимы[править]
Антонимы[править]
- —
Гиперонимы[править]
- ткань
Гипонимы[править]
- красный костный мозг, жёлтый костный мозг, слизистый костный мозг
Этимология[править]
От костный (кость) + мозг (см. этимологию этих слов).
Перевод[править]
| Список переводов | |
|
Библиография[править]
From Wikipedia, the free encyclopedia
| Bone marrow | |
|---|---|

A section of bone marrow tissue |
|
| Details | |
| System | Hematopoietic system Immune system[1] Lymphatic system |
| Identifiers | |
| Latin | Medulla ossium |
| MeSH | D001853 |
| TA98 | A13.1.01.001 |
| TA2 | 388 |
| FMA | 9608 |
| Anatomical terminology
[edit on Wikidata] |
Bone marrow is a semi-solid tissue found within the spongy (also known as cancellous) portions of bones.[2] In birds and mammals, bone marrow is the primary site of new blood cell production (or haematopoiesis).[3] It is composed of hematopoietic cells,[4] marrow adipose tissue, and supportive stromal cells. In adult humans, bone marrow is primarily located in the ribs, vertebrae, sternum, and bones of the pelvis.[5] Bone marrow comprises approximately 5% of total body mass in healthy adult humans, such that a man weighing 73 kg (161 lbs) will have around 3.7 kg (8 lbs) of bone marrow.[6]
Human marrow produces approximately 500 billion blood cells per day, which join the systemic circulation via permeable vasculature sinusoids within the medullary cavity.[7] All types of hematopoietic cells, including both myeloid and lymphoid lineages, are created in bone marrow; however, lymphoid cells must migrate to other lymphoid organs (e.g. thymus) in order to complete maturation.
Bone marrow transplants can be conducted to treat severe diseases of the bone marrow, including certain forms of cancer such as leukemia. Several types of stem cells are related to bone marrow. Hematopoietic stem cells in the bone marrow can give rise to hematopoietic lineage cells, and mesenchymal stem cells, which can be isolated from the primary culture of bone marrow stroma, can give rise to bone, adipose, and cartilage tissue.[8]
Structure[edit]
The composition of marrow is dynamic, as the mixture of cellular and non-cellular components (connective tissue) shifts with age and in response to systemic factors. In humans, marrow is colloquially characterized as «red» or «yellow» marrow (Latin: medulla ossium rubra, Latin: medulla ossium flava, respectively) depending on the prevalence of hematopoietic cells vs fat cells. While the precise mechanisms underlying marrow regulation are not understood,[7] compositional changes occur according to stereotypical patterns.[9] For example, a newborn baby’s bones exclusively contain hematopoietically active «red» marrow, and there is a progressive conversion towards «yellow» marrow with age. In adults, red marrow is found mainly in the central skeleton, such as the pelvis, sternum, cranium, ribs, vertebrae and scapulae, and variably found in the proximal epiphyseal ends of long bones such as the femur and humerus. In circumstances of chronic hypoxia, the body can convert yellow marrow back to red marrow to increase blood cell production.[10]
Hematopoietic components[edit]
At the cellular level, the main functional component of bone marrow includes the progenitor cells which are destined to mature into blood and lymphoid cells. Human marrow produces approximately 500 billion blood cells per day.[11] Marrow contains hematopoietic stem cells which give rise to the three classes of blood cells that are found in circulation: white blood cells (leukocytes), red blood cells (erythrocytes), and platelets (thrombocytes).[12]
| Group | Cell type | Average fraction |
Reference range |
|---|---|---|---|
| Myelopoietic cells |
Myeloblasts | 0.9 | 0.2–1.5 |
| Promyelocytes | 3.3% | 2.1–4.1 | |
| Neutrophilic myelocytes | 12.7% | 8.2–15.7 | |
| Eosinophilic myelocytes | 0.8% | 0.2–1.3 | |
| Neutrophilic metamyelocytes | 15.9% | 9.6–24.6 | |
| Eosinophilic metamyelocytes | 1.2% | 0.4–2.2 | |
| Neutrophilic band cells | 12.4% | 9.5–15.3 | |
| Eosinophilic band cells | 0.9% | 0.2–2.4 | |
| Segmented neutrophils | 7.4% | 6.0–12.0 | |
| Segmented eosinophils | 0.5% | 0.0–1.3 | |
| Segmented basophils and mast cells | 0.1% | 0.0–0.2 | |
| Erythropoietic cells |
Pronormoblasts | 0.6% | 0.2–1.3 |
| Basophilic normoblasts | 1.4% | 0.5–2.4 | |
| Polychromatic normoblasts | 21.6% | 17.9–29.2 | |
| Orthochromatic normoblast | 2.0% | 0.4–4.6 | |
| Other cell types |
Megakaryocytes | < 0.1% | 0.0-0.4 |
| Plasma cells | 1.3% | 0.4-3.9 | |
| Reticular cells | 0.3% | 0.0-0.9 | |
| Lymphocytes | 16.2% | 11.1-23.2 | |
| Monocytes | 0.3% | 0.0-0.8 |
Stroma[edit]
The stroma of the bone marrow includes all tissue not directly involved in the marrow’s primary function of hematopoiesis.[7] Stromal cells may be indirectly involved in hematopoiesis, providing a microenvironment that influences the function and differentiation of hematopoietic cells. For instance, they generate colony stimulating factors, which have a significant effect on hematopoiesis. Cell types that constitute the bone marrow stroma include:
- fibroblasts (reticular connective tissue)
- macrophages, which contribute especially to red blood cell production, as they deliver iron for hemoglobin production.
- adipocytes (fat cells)
- osteoblasts (synthesize bone)
- osteoclasts (resorb bone)
- endothelial cells, which form the sinusoids. These derive from endothelial stem cells, which are also present in the bone marrow.[12]
Function[edit]
Mesenchymal stem cells[edit]
The bone marrow stroma contains mesenchymal stem cells (MSCs),[12] which are also known as marrow stromal cells. These are multipotent stem cells that can differentiate into a variety of cell types. MSCs have been shown to differentiate, in vitro or in vivo, into osteoblasts, chondrocytes, myocytes, marrow adipocytes and beta-pancreatic islets cells.[citation needed]
Bone marrow barrier[edit]
The blood vessels of the bone marrow constitute a barrier, inhibiting immature blood cells from leaving the marrow. Only mature blood cells contain the membrane proteins, such as aquaporin and glycophorin, that are required to attach to and pass the blood vessel endothelium.[14] Hematopoietic stem cells may also cross the bone marrow barrier, and may thus be harvested from blood.[citation needed]
Lymphatic role[edit]
The red bone marrow is a key element of the lymphatic system, being one of the primary lymphoid organs that generate lymphocytes from immature hematopoietic progenitor cells.[15] The bone marrow and thymus constitute the primary lymphoid tissues involved in the production and early selection of lymphocytes. Furthermore, bone marrow performs a valve-like function to prevent the backflow of lymphatic fluid in the lymphatic system.[citation needed]
Compartmentalization[edit]
Biological compartmentalization is evident within the bone marrow, in that certain cell types tend to aggregate in specific areas. For instance, erythrocytes, macrophages, and their precursors tend to gather around blood vessels, while granulocytes gather at the borders of the bone marrow.[12]
As food[edit]
People have used animal bone-marrow in cuisine worldwide for millennia, as in the famed Milanese Ossobuco.[16]
Marrow oil[edit]
An 1892 text provides the following instructions and comments on hair oil:
Marrow oil.-
- Clarified beef marrow …….. 1+1⁄2oz.
- Oil of almonds ……………….. 1⁄4 pt.
Melt them together, and scent the mixture at will. Held in high repute as a hair oil by many. That of the shops has seldom any marrow in it, but lard instead. The appropriate scents are the same as for bears’ grease. It is generally tinged slightly yellow by means of a little palm oil or annatto.[17]
Clinical significance[edit]
Disease[edit]
The normal bone marrow architecture can be damaged or displaced by aplastic anemia, malignancies such as multiple myeloma, or infections such as tuberculosis, leading to a decrease in the production of blood cells and blood platelets. The bone marrow can also be affected by various forms of leukemia, which attacks its hematologic progenitor cells.[18] Furthermore, exposure to radiation or chemotherapy will kill many of the rapidly dividing cells of the bone marrow, and will therefore result in a depressed immune system. Many of the symptoms of radiation poisoning are due to damage sustained by the bone marrow cells.[citation needed]
To diagnose diseases involving the bone marrow, a bone marrow aspiration is sometimes performed. This typically involves using a hollow needle to acquire a sample of red bone marrow from the crest of the ilium under general or local anesthesia.[19]
Application of stem cells in therapeutics[edit]
Bone marrow derived stem cells have a wide array of application in regenerative medicine.[20]
Imaging[edit]
Medical imaging may provide a limited amount of information regarding bone marrow. Plain film x-rays pass through soft tissues such as marrow and do not provide visualization, although any changes in the structure of the associated bone may be detected.[21] CT imaging has somewhat better capacity for assessing the marrow cavity of bones, although with low sensitivity and specificity. For example, normal fatty «yellow» marrow in adult long bones is of low density (-30 to -100 Hounsfield units), between subcutaneous fat and soft tissue. Tissue with increased cellular composition, such as normal «red» marrow or cancer cells within the medullary cavity will measure variably higher in density.[22]
MRI is more sensitive and specific for assessing bone composition. MRI enables assessment of the average molecular composition of soft tissues and thus provides information regarding the relative fat content of marrow. In adult humans, «yellow» fatty marrow is the dominant tissue in bones, particularly in the (peripheral) appendicular skeleton. Because fat molecules have a high T1-relaxivity, T1-weighted imaging sequences show «yellow» fatty marrow as bright (hyperintense). Furthermore, normal fatty marrow loses signal on fat-saturation sequences, in a similar pattern to subcutaneous fat.[citation needed]
When «yellow» fatty marrow becomes replaced by tissue with more cellular composition, this change is apparent as decreased brightness on T1-weighted sequences. Both normal «red» marrow and pathologic marrow lesions (such as cancer) are darker than «yellow» marrow on T1-weight sequences, although can often be distinguished by comparison with the MR signal intensity of adjacent soft tissues. Normal «red» marrow is typically equivalent or brighter than skeletal muscle or intervertebral disc on T1-weighted sequences.[9][23]
Fatty marrow change, the inverse of red marrow hyperplasia, can occur with normal aging,[24] though it can also be seen with certain treatments such as radiation therapy. Diffuse marrow T1 hypointensity without contrast enhancement or cortical discontinuity suggests red marrow conversion or myelofibrosis. Falsely normal marrow on T1 can be seen with diffuse multiple myeloma or leukemic infiltration when the water to fat ratio is not sufficiently altered, as may be seen with lower grade tumors or earlier in the disease process.[25]
Histology[edit]
Bone marrow examination is the pathologic analysis of samples of bone marrow obtained via biopsy and bone marrow aspiration. Bone marrow examination is used in the diagnosis of a number of conditions, including leukemia, multiple myeloma, anemia, and pancytopenia. The bone marrow produces the cellular elements of the blood, including platelets, red blood cells and white blood cells. While much information can be gleaned by testing the blood itself (drawn from a vein by phlebotomy), it is sometimes necessary to examine the source of the blood cells in the bone marrow to obtain more information on hematopoiesis; this is the role of bone marrow aspiration and biopsy.[citation needed]
The ratio between myeloid series and erythroid cells is relevant to bone marrow function, and also to diseases of the bone marrow and peripheral blood, such as leukemia and anemia. The normal myeloid-to-erythroid ratio is around 3:1; this ratio may increase in myelogenous leukemias, decrease in polycythemias, and reverse in cases of thalassemia.[26]
Donation and transplantation[edit]
A bone marrow harvest in progress.
The preferred sites for the procedure
In a bone marrow transplant, hematopoietic stem cells are removed from a person and infused into another person (allogenic) or into the same person at a later time (autologous). If the donor and recipient are compatible, these infused cells will then travel to the bone marrow and initiate blood cell production. Transplantation from one person to another is conducted for the treatment of severe bone marrow diseases, such as congenital defects, autoimmune diseases or malignancies. The patient’s own marrow is first killed off with drugs or radiation, and then the new stem cells are introduced. Before radiation therapy or chemotherapy in cases of cancer, some of the patient’s hematopoietic stem cells are sometimes harvested and later infused back when the therapy is finished to restore the immune system.[27]
Bone marrow stem cells can be induced to become neural cells to treat neurological illnesses,[28] and can also potentially be used for the treatment of other illnesses, such as inflammatory bowel disease.[29] In 2013, following a clinical trial, scientists proposed that bone marrow transplantation could be used to treat HIV in conjunction with antiretroviral drugs;[30][31] however, it was later found that HIV remained in the bodies of the test subjects.[32]
Harvesting[edit]
The stem cells are typically harvested directly from the red marrow in the iliac crest, often under general anesthesia. The procedure is minimally invasive and does not require stitches afterwards. Depending on the donor’s health and reaction to the procedure, the actual harvesting can be an outpatient procedure, or can require 1–2 days of recovery in the hospital.[33]
Another option is to administer certain drugs that stimulate the release of stem cells from the bone marrow into circulating blood.[34] An intravenous catheter is inserted into the donor’s arm, and the stem cells are then filtered out of the blood. This procedure is similar to that used in blood or platelet donation. In adults, bone marrow may also be taken from the sternum, while the tibia is often used when taking samples from infants.[19] In newborns, stem cells may be retrieved from the umbilical cord.[35]
Persistent viruses[edit]
Using quantitative Polymerase Chain Reaction (qPCR) and Next-generation Sequencing (NGS) a maximum of five DNA viruses per individual have been identified. Included were several herpesviruses, hepatitis B virus, Merkel cell polyomavirus, and human papillomavirus 31. Given the reactivation and/or oncogenic potential of these viruses, their repercussion on hematopoietic and malignant disorders calls for further studies.[36]
Fossil record[edit]
Bone marrow may have first evolved in Eusthenopteron, a species of prehistoric fish with close links to early tetrapods.
The earliest fossilised evidence of bone marrow was discovered in 2014 in Eusthenopteron, a lobe-finned fish which lived during the Devonian period approximately 370 million years ago.[37] Scientists from Uppsala University and the European Synchrotron Radiation Facility used X-ray synchrotron microtomography to study the fossilised interior of the skeleton’s humerus, finding organised tubular structures akin to modern vertebrate bone marrow.[37] Eusthenopteron is closely related to the early tetrapods, which ultimately evolved into the land-dwelling mammals and lizards of the present day.[37]
See also[edit]
- LOC100272216 protein
- Myelonecrosis
- National Marrow Donor Program
- Gift of Life Marrow Registry
References[edit]
- ^ Schmidt, Richard F.; Lang, Florian; Heckmann, Manfred (30 November 2010). What are the organs of the immune system?. Institute for Quality and Efficiency in Health Care. pp. 3/7.
- ^ C., Farhi, Diane (2009). Pathology of bone marrow and blood cells (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott William & Wilkins. ISBN 9780781770934. OCLC 191807944.
- ^ Arikan, Hüseyin; Çiçek, Kerim (2014). «Haematology of amphibians and reptiles: a review» (PDF). North-Western Journal of Zoology. 10: 190–209.
- ^ Monga I, Kaur K, Dhanda S (March 2022). «Revisiting hematopoiesis: applications of the bulk and single-cell transcriptomics dissecting transcriptional heterogeneity in hematopoietic stem cells». Briefings in Functional Genomics. 21 (3): 159–176. doi:10.1093/bfgp/elac002. PMID 35265979.
- ^ Katherine, Abel (2013). Official CPC Certification Study Guide. American Medical Association.
- ^ Hindorf, C.; Glatting, G.; Chiesa, C.; Lindén, O.; Flux, G. (2010). «EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry». Eur J Nucl Med Mol Imaging. 37 (6): 1238–1250. doi:10.1007/s00259-010-1422-4. PMID 20411259. S2CID 9755621.
- ^ a b c Birbrair, Alexander; Frenette, Paul S. (1 March 2016). «Niche heterogeneity in the bone marrow». Annals of the New York Academy of Sciences. 1370 (1): 82–96. Bibcode:2016NYASA1370…82B. doi:10.1111/nyas.13016. ISSN 1749-6632. PMC 4938003. PMID 27015419.
- ^ Lindberg, Matthew R.; Lamps, Laura W. (2018). «Bone Marrow». Diagnostic Pathology: Normal Histology. pp. 130–137. doi:10.1016/B978-0-323-54803-8.50035-8. ISBN 9780323548038.
- ^ a b Chan, Brian Y.; Gill, Kara G.; Rebsamen, Susan L.; Nguyen, Jie C. (1 October 2016). «MR Imaging of Pediatric Bone Marrow». RadioGraphics. 36 (6): 1911–1930. doi:10.1148/rg.2016160056. ISSN 0271-5333. PMID 27726743.
- ^ Poulton, T B; Murphy, W D; Duerk, J L; Chapek, C C; Feiglin, D H (1 December 1993). «Bone marrow reconversion in adults who are smokers: MR Imaging findings». American Journal of Roentgenology. 161 (6): 1217–1221. doi:10.2214/ajr.161.6.8249729. ISSN 0361-803X. PMID 8249729.
- ^ Nombela-Arrieta, Cesar; G. Manz, Markus (2017). «Quantification and three-dimensional microanatomical organization of the bone marrow». Blood Advances. 1 (6): 407–416. doi:10.1182/bloodadvances.2016003194. PMC 5738992. PMID 29296956.
- ^ a b c d Raphael Rubin & David S. Strayer (2007). Rubin’s Pathology: Clinicopathologic Foundations of Medicine. Lippincott Williams & Wilkins. p. 90. ISBN 978-0-7817-9516-6.
- ^ Appendix A:IV in Wintrobe’s clinical hematology (9th edition). Philadelphia: Lea & Febiger (1993).
- ^ «The Red Cell Membrane: structure and pathologies» (PDF). Australian Centre for Blood Diseases/Monash University. Retrieved 24 January 2015.
- ^ The Lymphatic System. Allonhealth.com. Retrieved 5 December 2011.
- ^
Fabricant, Florence. «Begging for Bones: A New Craving for Marrow». The New York Times. September 16, 1998. - ^
Hopkins, Albert Allis, ed. (1892). «Hair». The Scientific American Cyclopedia of Receipts, Notes and Queries. New York: Munn & Company. p. 255. Retrieved 18 December 2022. - ^ Bonnet, D; Dick, JE (1997). «Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell». Nature Medicine. 3 (7): 730–737. doi:10.1038/nm0797-730. PMID 9212098. S2CID 205381050.
- ^ a b «Bone Marrow Aspiration and Biopsy». Lab Tests Online UK. Retrieved 16 February 2013.
- ^
Mahla RS (2016). «Stem cells application in regenerative medicine and disease threpeutics». International Journal of Cell Biology. 2016 (7): 1–24. doi:10.1155/2016/6940283. PMC 4969512. PMID 27516776.{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ Ellmann, Stephan; Beck, Michael; Kuwert, Torsten; Uder, Michael; Bäuerle, Tobias (2015). «Multimodal imaging of bone metastases: From preclinical to clinical applications». Journal of Orthopaedic Translation. 3 (4): 166–177. doi:10.1016/j.jot.2015.07.004. PMC 5986987. PMID 30035055.
- ^ Nishida, Y; Matsue, Y; Suehara, Y; Fukumoto, K; Fujisawa, M; Takeuchi, M; Ouchi, E; Matsue, K (August 2015). «Clinical and prognostic significance of bone marrow abnormalities in the appendicular skeleton detected by low-doe whole-body multidetector computed tomography in patients with multiple myeloma». Blood Cancer Journal. 5 (7): e329. doi:10.1038/bcj.2015.57. ISSN 2044-5385. PMC 4526783. PMID 26230953.
- ^ Poulton, TB; Murphy, WD; Duerk, JL; Chapek, CC; Feiglin, DH (December 1993). «Bone marrow reconversion in adults who are smokers: MR Imaging findings». AJR. American Journal of Roentgenology. 161 (6): 1217–21. doi:10.2214/ajr.161.6.8249729. PMID 8249729.
- ^ Shah, LM; Hanrahan, CJ (December 2011). «MRI of spinal bone marrow: part I, techniques and normal age-related appearances». AJR. American Journal of Roentgenology. 197 (6): 1298–308. doi:10.2214/ajr.11.7005. PMID 22109283. S2CID 20115888.
- ^ Vande Berg, BC; Lecouvet, FE; Galant, C; Maldague, BE; Malghem, J (July 2005). «Normal variants and frequent marrow alterations that simulate bone marrow lesions at MR imaging». Radiologic Clinics of North America. 43 (4): 761–70, ix. doi:10.1016/j.rcl.2005.01.007. PMID 15893536.
- ^ «Definition: ‘M:E Ratio’«. Stedman’s Medical Dictionary via MediLexicon.com. 2006. Archived from the original on 10 May 2013. Retrieved 20 December 2012.
- ^ «Bone marrow transplantation». UpToDate.com. Retrieved 12 April 2014.
- ^ «Antibody Transforms Stem Cells Directly Into Brain Cells». Science Daily. 22 April 2013. Retrieved 24 April 2013.
- ^ «Research Supports Promise of Cell Therapy for Bowel Disease». Wake Forest Baptist Medical Center. 28 February 2013. Retrieved 5 March 2013.
- ^ «Bone marrow ‘frees men of HIV drugs’«. BBC. 3 July 2013. Retrieved 3 July 2013.
- ^ «Stem-Cell Transplants Erase HIV In Two Men». PopSci. 3 July 2013. Retrieved 3 July 2013.
- ^ «HIV Returns in Two Men Thought ‘Cured’ by Bone Marrow Transplants». RH Reality Check. 10 December 2013. Retrieved 10 December 2013.
- ^ National Marrow Donor Program Donor Guide Archived 8 September 2008 at the Wayback Machine. Marrow.org. Retrieved 5 November 2012.
- ^ Bone marrow donation: What to expect when you donate. Mayo Clinic. Retrieved 16 February 2013.
- ^ McGuckin, C. P.; Forraz, N.; Baradez, M. -O.; Navran, S.; Zhao, J.; Urban, R.; Tilton, R.; Denner, L. (2005). «Production of stem cells with embryonic characteristics from human umbilical cord blood». Cell Proliferation. 38 (4): 245–255. doi:10.1111/j.1365-2184.2005.00346.x. PMC 6496335. PMID 16098183.
- ^ Toppinen, Mari; Sajantila, Antti; Pratas, Diogo; Hedman, Klaus; Perdomo, Maria F. (2021). «The Human Bone Marrow Is Host to the DNAs of Several Viruses». Front. Cell. Infect. Microbiol. 11: 657245. doi:10.3389/fcimb.2021.657245. PMC 8100435. PMID 33968803.
- ^ a b c Sanchez, S.; Tafforeau, P.; Ahlberg, P. E. (2014). «The humerus of Eusthenopteron: a puzzling organization presaging the establishment of tetrapod limb bone marrow». Proceedings of the Royal Society B: Biological Sciences. 281 (1782): 20140299. doi:10.1098/rspb.2014.0299. PMC 3973280. PMID 24648231.
Further reading[edit]
- Nature Bone Marrow Transplantation (Nature Publishing Group) – specialist scientific journal with articles on bone marrow biology and clinical uses.
- Cooper, B (2011). «The origins of bone marrow as the seedbed of our blood: from antiquity to the time of Osler». Baylor University Medical Center Proceedings. 24 (2): 115–8. doi:10.1080/08998280.2011.11928697. PMC 3069519. PMID 21566758.
- Wang J, Liu X, Lu H, Jiang C, Cui X, Yu L, Fu X, Li Q, Wang J (2015). «CXCR4(+)CD45(-) BMMNC subpopulation is superior to unfractionated BMMNCs for protection after ischemic stroke in mice». Brain Behav. Immun. 45: 98–108. doi:10.1016/j.bbi.2014.12.015. PMC 4342301. PMID 25526817.
External links[edit]
- Bone marrow histology photomicrographs
From Wikipedia, the free encyclopedia
| Bone marrow | |
|---|---|

A section of bone marrow tissue |
|
| Details | |
| System | Hematopoietic system Immune system[1] Lymphatic system |
| Identifiers | |
| Latin | Medulla ossium |
| MeSH | D001853 |
| TA98 | A13.1.01.001 |
| TA2 | 388 |
| FMA | 9608 |
| Anatomical terminology
[edit on Wikidata] |
Bone marrow is a semi-solid tissue found within the spongy (also known as cancellous) portions of bones.[2] In birds and mammals, bone marrow is the primary site of new blood cell production (or haematopoiesis).[3] It is composed of hematopoietic cells,[4] marrow adipose tissue, and supportive stromal cells. In adult humans, bone marrow is primarily located in the ribs, vertebrae, sternum, and bones of the pelvis.[5] Bone marrow comprises approximately 5% of total body mass in healthy adult humans, such that a man weighing 73 kg (161 lbs) will have around 3.7 kg (8 lbs) of bone marrow.[6]
Human marrow produces approximately 500 billion blood cells per day, which join the systemic circulation via permeable vasculature sinusoids within the medullary cavity.[7] All types of hematopoietic cells, including both myeloid and lymphoid lineages, are created in bone marrow; however, lymphoid cells must migrate to other lymphoid organs (e.g. thymus) in order to complete maturation.
Bone marrow transplants can be conducted to treat severe diseases of the bone marrow, including certain forms of cancer such as leukemia. Several types of stem cells are related to bone marrow. Hematopoietic stem cells in the bone marrow can give rise to hematopoietic lineage cells, and mesenchymal stem cells, which can be isolated from the primary culture of bone marrow stroma, can give rise to bone, adipose, and cartilage tissue.[8]
Structure[edit]
The composition of marrow is dynamic, as the mixture of cellular and non-cellular components (connective tissue) shifts with age and in response to systemic factors. In humans, marrow is colloquially characterized as «red» or «yellow» marrow (Latin: medulla ossium rubra, Latin: medulla ossium flava, respectively) depending on the prevalence of hematopoietic cells vs fat cells. While the precise mechanisms underlying marrow regulation are not understood,[7] compositional changes occur according to stereotypical patterns.[9] For example, a newborn baby’s bones exclusively contain hematopoietically active «red» marrow, and there is a progressive conversion towards «yellow» marrow with age. In adults, red marrow is found mainly in the central skeleton, such as the pelvis, sternum, cranium, ribs, vertebrae and scapulae, and variably found in the proximal epiphyseal ends of long bones such as the femur and humerus. In circumstances of chronic hypoxia, the body can convert yellow marrow back to red marrow to increase blood cell production.[10]
Hematopoietic components[edit]
At the cellular level, the main functional component of bone marrow includes the progenitor cells which are destined to mature into blood and lymphoid cells. Human marrow produces approximately 500 billion blood cells per day.[11] Marrow contains hematopoietic stem cells which give rise to the three classes of blood cells that are found in circulation: white blood cells (leukocytes), red blood cells (erythrocytes), and platelets (thrombocytes).[12]
| Group | Cell type | Average fraction |
Reference range |
|---|---|---|---|
| Myelopoietic cells |
Myeloblasts | 0.9 | 0.2–1.5 |
| Promyelocytes | 3.3% | 2.1–4.1 | |
| Neutrophilic myelocytes | 12.7% | 8.2–15.7 | |
| Eosinophilic myelocytes | 0.8% | 0.2–1.3 | |
| Neutrophilic metamyelocytes | 15.9% | 9.6–24.6 | |
| Eosinophilic metamyelocytes | 1.2% | 0.4–2.2 | |
| Neutrophilic band cells | 12.4% | 9.5–15.3 | |
| Eosinophilic band cells | 0.9% | 0.2–2.4 | |
| Segmented neutrophils | 7.4% | 6.0–12.0 | |
| Segmented eosinophils | 0.5% | 0.0–1.3 | |
| Segmented basophils and mast cells | 0.1% | 0.0–0.2 | |
| Erythropoietic cells |
Pronormoblasts | 0.6% | 0.2–1.3 |
| Basophilic normoblasts | 1.4% | 0.5–2.4 | |
| Polychromatic normoblasts | 21.6% | 17.9–29.2 | |
| Orthochromatic normoblast | 2.0% | 0.4–4.6 | |
| Other cell types |
Megakaryocytes | < 0.1% | 0.0-0.4 |
| Plasma cells | 1.3% | 0.4-3.9 | |
| Reticular cells | 0.3% | 0.0-0.9 | |
| Lymphocytes | 16.2% | 11.1-23.2 | |
| Monocytes | 0.3% | 0.0-0.8 |
Stroma[edit]
The stroma of the bone marrow includes all tissue not directly involved in the marrow’s primary function of hematopoiesis.[7] Stromal cells may be indirectly involved in hematopoiesis, providing a microenvironment that influences the function and differentiation of hematopoietic cells. For instance, they generate colony stimulating factors, which have a significant effect on hematopoiesis. Cell types that constitute the bone marrow stroma include:
- fibroblasts (reticular connective tissue)
- macrophages, which contribute especially to red blood cell production, as they deliver iron for hemoglobin production.
- adipocytes (fat cells)
- osteoblasts (synthesize bone)
- osteoclasts (resorb bone)
- endothelial cells, which form the sinusoids. These derive from endothelial stem cells, which are also present in the bone marrow.[12]
Function[edit]
Mesenchymal stem cells[edit]
The bone marrow stroma contains mesenchymal stem cells (MSCs),[12] which are also known as marrow stromal cells. These are multipotent stem cells that can differentiate into a variety of cell types. MSCs have been shown to differentiate, in vitro or in vivo, into osteoblasts, chondrocytes, myocytes, marrow adipocytes and beta-pancreatic islets cells.[citation needed]
Bone marrow barrier[edit]
The blood vessels of the bone marrow constitute a barrier, inhibiting immature blood cells from leaving the marrow. Only mature blood cells contain the membrane proteins, such as aquaporin and glycophorin, that are required to attach to and pass the blood vessel endothelium.[14] Hematopoietic stem cells may also cross the bone marrow barrier, and may thus be harvested from blood.[citation needed]
Lymphatic role[edit]
The red bone marrow is a key element of the lymphatic system, being one of the primary lymphoid organs that generate lymphocytes from immature hematopoietic progenitor cells.[15] The bone marrow and thymus constitute the primary lymphoid tissues involved in the production and early selection of lymphocytes. Furthermore, bone marrow performs a valve-like function to prevent the backflow of lymphatic fluid in the lymphatic system.[citation needed]
Compartmentalization[edit]
Biological compartmentalization is evident within the bone marrow, in that certain cell types tend to aggregate in specific areas. For instance, erythrocytes, macrophages, and their precursors tend to gather around blood vessels, while granulocytes gather at the borders of the bone marrow.[12]
As food[edit]
People have used animal bone-marrow in cuisine worldwide for millennia, as in the famed Milanese Ossobuco.[16]
Marrow oil[edit]
An 1892 text provides the following instructions and comments on hair oil:
Marrow oil.-
- Clarified beef marrow …….. 1+1⁄2oz.
- Oil of almonds ……………….. 1⁄4 pt.
Melt them together, and scent the mixture at will. Held in high repute as a hair oil by many. That of the shops has seldom any marrow in it, but lard instead. The appropriate scents are the same as for bears’ grease. It is generally tinged slightly yellow by means of a little palm oil or annatto.[17]
Clinical significance[edit]
Disease[edit]
The normal bone marrow architecture can be damaged or displaced by aplastic anemia, malignancies such as multiple myeloma, or infections such as tuberculosis, leading to a decrease in the production of blood cells and blood platelets. The bone marrow can also be affected by various forms of leukemia, which attacks its hematologic progenitor cells.[18] Furthermore, exposure to radiation or chemotherapy will kill many of the rapidly dividing cells of the bone marrow, and will therefore result in a depressed immune system. Many of the symptoms of radiation poisoning are due to damage sustained by the bone marrow cells.[citation needed]
To diagnose diseases involving the bone marrow, a bone marrow aspiration is sometimes performed. This typically involves using a hollow needle to acquire a sample of red bone marrow from the crest of the ilium under general or local anesthesia.[19]
Application of stem cells in therapeutics[edit]
Bone marrow derived stem cells have a wide array of application in regenerative medicine.[20]
Imaging[edit]
Medical imaging may provide a limited amount of information regarding bone marrow. Plain film x-rays pass through soft tissues such as marrow and do not provide visualization, although any changes in the structure of the associated bone may be detected.[21] CT imaging has somewhat better capacity for assessing the marrow cavity of bones, although with low sensitivity and specificity. For example, normal fatty «yellow» marrow in adult long bones is of low density (-30 to -100 Hounsfield units), between subcutaneous fat and soft tissue. Tissue with increased cellular composition, such as normal «red» marrow or cancer cells within the medullary cavity will measure variably higher in density.[22]
MRI is more sensitive and specific for assessing bone composition. MRI enables assessment of the average molecular composition of soft tissues and thus provides information regarding the relative fat content of marrow. In adult humans, «yellow» fatty marrow is the dominant tissue in bones, particularly in the (peripheral) appendicular skeleton. Because fat molecules have a high T1-relaxivity, T1-weighted imaging sequences show «yellow» fatty marrow as bright (hyperintense). Furthermore, normal fatty marrow loses signal on fat-saturation sequences, in a similar pattern to subcutaneous fat.[citation needed]
When «yellow» fatty marrow becomes replaced by tissue with more cellular composition, this change is apparent as decreased brightness on T1-weighted sequences. Both normal «red» marrow and pathologic marrow lesions (such as cancer) are darker than «yellow» marrow on T1-weight sequences, although can often be distinguished by comparison with the MR signal intensity of adjacent soft tissues. Normal «red» marrow is typically equivalent or brighter than skeletal muscle or intervertebral disc on T1-weighted sequences.[9][23]
Fatty marrow change, the inverse of red marrow hyperplasia, can occur with normal aging,[24] though it can also be seen with certain treatments such as radiation therapy. Diffuse marrow T1 hypointensity without contrast enhancement or cortical discontinuity suggests red marrow conversion or myelofibrosis. Falsely normal marrow on T1 can be seen with diffuse multiple myeloma or leukemic infiltration when the water to fat ratio is not sufficiently altered, as may be seen with lower grade tumors or earlier in the disease process.[25]
Histology[edit]
Bone marrow examination is the pathologic analysis of samples of bone marrow obtained via biopsy and bone marrow aspiration. Bone marrow examination is used in the diagnosis of a number of conditions, including leukemia, multiple myeloma, anemia, and pancytopenia. The bone marrow produces the cellular elements of the blood, including platelets, red blood cells and white blood cells. While much information can be gleaned by testing the blood itself (drawn from a vein by phlebotomy), it is sometimes necessary to examine the source of the blood cells in the bone marrow to obtain more information on hematopoiesis; this is the role of bone marrow aspiration and biopsy.[citation needed]
The ratio between myeloid series and erythroid cells is relevant to bone marrow function, and also to diseases of the bone marrow and peripheral blood, such as leukemia and anemia. The normal myeloid-to-erythroid ratio is around 3:1; this ratio may increase in myelogenous leukemias, decrease in polycythemias, and reverse in cases of thalassemia.[26]
Donation and transplantation[edit]
A bone marrow harvest in progress.
The preferred sites for the procedure
In a bone marrow transplant, hematopoietic stem cells are removed from a person and infused into another person (allogenic) or into the same person at a later time (autologous). If the donor and recipient are compatible, these infused cells will then travel to the bone marrow and initiate blood cell production. Transplantation from one person to another is conducted for the treatment of severe bone marrow diseases, such as congenital defects, autoimmune diseases or malignancies. The patient’s own marrow is first killed off with drugs or radiation, and then the new stem cells are introduced. Before radiation therapy or chemotherapy in cases of cancer, some of the patient’s hematopoietic stem cells are sometimes harvested and later infused back when the therapy is finished to restore the immune system.[27]
Bone marrow stem cells can be induced to become neural cells to treat neurological illnesses,[28] and can also potentially be used for the treatment of other illnesses, such as inflammatory bowel disease.[29] In 2013, following a clinical trial, scientists proposed that bone marrow transplantation could be used to treat HIV in conjunction with antiretroviral drugs;[30][31] however, it was later found that HIV remained in the bodies of the test subjects.[32]
Harvesting[edit]
The stem cells are typically harvested directly from the red marrow in the iliac crest, often under general anesthesia. The procedure is minimally invasive and does not require stitches afterwards. Depending on the donor’s health and reaction to the procedure, the actual harvesting can be an outpatient procedure, or can require 1–2 days of recovery in the hospital.[33]
Another option is to administer certain drugs that stimulate the release of stem cells from the bone marrow into circulating blood.[34] An intravenous catheter is inserted into the donor’s arm, and the stem cells are then filtered out of the blood. This procedure is similar to that used in blood or platelet donation. In adults, bone marrow may also be taken from the sternum, while the tibia is often used when taking samples from infants.[19] In newborns, stem cells may be retrieved from the umbilical cord.[35]
Persistent viruses[edit]
Using quantitative Polymerase Chain Reaction (qPCR) and Next-generation Sequencing (NGS) a maximum of five DNA viruses per individual have been identified. Included were several herpesviruses, hepatitis B virus, Merkel cell polyomavirus, and human papillomavirus 31. Given the reactivation and/or oncogenic potential of these viruses, their repercussion on hematopoietic and malignant disorders calls for further studies.[36]
Fossil record[edit]
Bone marrow may have first evolved in Eusthenopteron, a species of prehistoric fish with close links to early tetrapods.
The earliest fossilised evidence of bone marrow was discovered in 2014 in Eusthenopteron, a lobe-finned fish which lived during the Devonian period approximately 370 million years ago.[37] Scientists from Uppsala University and the European Synchrotron Radiation Facility used X-ray synchrotron microtomography to study the fossilised interior of the skeleton’s humerus, finding organised tubular structures akin to modern vertebrate bone marrow.[37] Eusthenopteron is closely related to the early tetrapods, which ultimately evolved into the land-dwelling mammals and lizards of the present day.[37]
See also[edit]
- LOC100272216 protein
- Myelonecrosis
- National Marrow Donor Program
- Gift of Life Marrow Registry
References[edit]
- ^ Schmidt, Richard F.; Lang, Florian; Heckmann, Manfred (30 November 2010). What are the organs of the immune system?. Institute for Quality and Efficiency in Health Care. pp. 3/7.
- ^ C., Farhi, Diane (2009). Pathology of bone marrow and blood cells (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott William & Wilkins. ISBN 9780781770934. OCLC 191807944.
- ^ Arikan, Hüseyin; Çiçek, Kerim (2014). «Haematology of amphibians and reptiles: a review» (PDF). North-Western Journal of Zoology. 10: 190–209.
- ^ Monga I, Kaur K, Dhanda S (March 2022). «Revisiting hematopoiesis: applications of the bulk and single-cell transcriptomics dissecting transcriptional heterogeneity in hematopoietic stem cells». Briefings in Functional Genomics. 21 (3): 159–176. doi:10.1093/bfgp/elac002. PMID 35265979.
- ^ Katherine, Abel (2013). Official CPC Certification Study Guide. American Medical Association.
- ^ Hindorf, C.; Glatting, G.; Chiesa, C.; Lindén, O.; Flux, G. (2010). «EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry». Eur J Nucl Med Mol Imaging. 37 (6): 1238–1250. doi:10.1007/s00259-010-1422-4. PMID 20411259. S2CID 9755621.
- ^ a b c Birbrair, Alexander; Frenette, Paul S. (1 March 2016). «Niche heterogeneity in the bone marrow». Annals of the New York Academy of Sciences. 1370 (1): 82–96. Bibcode:2016NYASA1370…82B. doi:10.1111/nyas.13016. ISSN 1749-6632. PMC 4938003. PMID 27015419.
- ^ Lindberg, Matthew R.; Lamps, Laura W. (2018). «Bone Marrow». Diagnostic Pathology: Normal Histology. pp. 130–137. doi:10.1016/B978-0-323-54803-8.50035-8. ISBN 9780323548038.
- ^ a b Chan, Brian Y.; Gill, Kara G.; Rebsamen, Susan L.; Nguyen, Jie C. (1 October 2016). «MR Imaging of Pediatric Bone Marrow». RadioGraphics. 36 (6): 1911–1930. doi:10.1148/rg.2016160056. ISSN 0271-5333. PMID 27726743.
- ^ Poulton, T B; Murphy, W D; Duerk, J L; Chapek, C C; Feiglin, D H (1 December 1993). «Bone marrow reconversion in adults who are smokers: MR Imaging findings». American Journal of Roentgenology. 161 (6): 1217–1221. doi:10.2214/ajr.161.6.8249729. ISSN 0361-803X. PMID 8249729.
- ^ Nombela-Arrieta, Cesar; G. Manz, Markus (2017). «Quantification and three-dimensional microanatomical organization of the bone marrow». Blood Advances. 1 (6): 407–416. doi:10.1182/bloodadvances.2016003194. PMC 5738992. PMID 29296956.
- ^ a b c d Raphael Rubin & David S. Strayer (2007). Rubin’s Pathology: Clinicopathologic Foundations of Medicine. Lippincott Williams & Wilkins. p. 90. ISBN 978-0-7817-9516-6.
- ^ Appendix A:IV in Wintrobe’s clinical hematology (9th edition). Philadelphia: Lea & Febiger (1993).
- ^ «The Red Cell Membrane: structure and pathologies» (PDF). Australian Centre for Blood Diseases/Monash University. Retrieved 24 January 2015.
- ^ The Lymphatic System. Allonhealth.com. Retrieved 5 December 2011.
- ^
Fabricant, Florence. «Begging for Bones: A New Craving for Marrow». The New York Times. September 16, 1998. - ^
Hopkins, Albert Allis, ed. (1892). «Hair». The Scientific American Cyclopedia of Receipts, Notes and Queries. New York: Munn & Company. p. 255. Retrieved 18 December 2022. - ^ Bonnet, D; Dick, JE (1997). «Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell». Nature Medicine. 3 (7): 730–737. doi:10.1038/nm0797-730. PMID 9212098. S2CID 205381050.
- ^ a b «Bone Marrow Aspiration and Biopsy». Lab Tests Online UK. Retrieved 16 February 2013.
- ^
Mahla RS (2016). «Stem cells application in regenerative medicine and disease threpeutics». International Journal of Cell Biology. 2016 (7): 1–24. doi:10.1155/2016/6940283. PMC 4969512. PMID 27516776.{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ Ellmann, Stephan; Beck, Michael; Kuwert, Torsten; Uder, Michael; Bäuerle, Tobias (2015). «Multimodal imaging of bone metastases: From preclinical to clinical applications». Journal of Orthopaedic Translation. 3 (4): 166–177. doi:10.1016/j.jot.2015.07.004. PMC 5986987. PMID 30035055.
- ^ Nishida, Y; Matsue, Y; Suehara, Y; Fukumoto, K; Fujisawa, M; Takeuchi, M; Ouchi, E; Matsue, K (August 2015). «Clinical and prognostic significance of bone marrow abnormalities in the appendicular skeleton detected by low-doe whole-body multidetector computed tomography in patients with multiple myeloma». Blood Cancer Journal. 5 (7): e329. doi:10.1038/bcj.2015.57. ISSN 2044-5385. PMC 4526783. PMID 26230953.
- ^ Poulton, TB; Murphy, WD; Duerk, JL; Chapek, CC; Feiglin, DH (December 1993). «Bone marrow reconversion in adults who are smokers: MR Imaging findings». AJR. American Journal of Roentgenology. 161 (6): 1217–21. doi:10.2214/ajr.161.6.8249729. PMID 8249729.
- ^ Shah, LM; Hanrahan, CJ (December 2011). «MRI of spinal bone marrow: part I, techniques and normal age-related appearances». AJR. American Journal of Roentgenology. 197 (6): 1298–308. doi:10.2214/ajr.11.7005. PMID 22109283. S2CID 20115888.
- ^ Vande Berg, BC; Lecouvet, FE; Galant, C; Maldague, BE; Malghem, J (July 2005). «Normal variants and frequent marrow alterations that simulate bone marrow lesions at MR imaging». Radiologic Clinics of North America. 43 (4): 761–70, ix. doi:10.1016/j.rcl.2005.01.007. PMID 15893536.
- ^ «Definition: ‘M:E Ratio’«. Stedman’s Medical Dictionary via MediLexicon.com. 2006. Archived from the original on 10 May 2013. Retrieved 20 December 2012.
- ^ «Bone marrow transplantation». UpToDate.com. Retrieved 12 April 2014.
- ^ «Antibody Transforms Stem Cells Directly Into Brain Cells». Science Daily. 22 April 2013. Retrieved 24 April 2013.
- ^ «Research Supports Promise of Cell Therapy for Bowel Disease». Wake Forest Baptist Medical Center. 28 February 2013. Retrieved 5 March 2013.
- ^ «Bone marrow ‘frees men of HIV drugs’«. BBC. 3 July 2013. Retrieved 3 July 2013.
- ^ «Stem-Cell Transplants Erase HIV In Two Men». PopSci. 3 July 2013. Retrieved 3 July 2013.
- ^ «HIV Returns in Two Men Thought ‘Cured’ by Bone Marrow Transplants». RH Reality Check. 10 December 2013. Retrieved 10 December 2013.
- ^ National Marrow Donor Program Donor Guide Archived 8 September 2008 at the Wayback Machine. Marrow.org. Retrieved 5 November 2012.
- ^ Bone marrow donation: What to expect when you donate. Mayo Clinic. Retrieved 16 February 2013.
- ^ McGuckin, C. P.; Forraz, N.; Baradez, M. -O.; Navran, S.; Zhao, J.; Urban, R.; Tilton, R.; Denner, L. (2005). «Production of stem cells with embryonic characteristics from human umbilical cord blood». Cell Proliferation. 38 (4): 245–255. doi:10.1111/j.1365-2184.2005.00346.x. PMC 6496335. PMID 16098183.
- ^ Toppinen, Mari; Sajantila, Antti; Pratas, Diogo; Hedman, Klaus; Perdomo, Maria F. (2021). «The Human Bone Marrow Is Host to the DNAs of Several Viruses». Front. Cell. Infect. Microbiol. 11: 657245. doi:10.3389/fcimb.2021.657245. PMC 8100435. PMID 33968803.
- ^ a b c Sanchez, S.; Tafforeau, P.; Ahlberg, P. E. (2014). «The humerus of Eusthenopteron: a puzzling organization presaging the establishment of tetrapod limb bone marrow». Proceedings of the Royal Society B: Biological Sciences. 281 (1782): 20140299. doi:10.1098/rspb.2014.0299. PMC 3973280. PMID 24648231.
Further reading[edit]
- Nature Bone Marrow Transplantation (Nature Publishing Group) – specialist scientific journal with articles on bone marrow biology and clinical uses.
- Cooper, B (2011). «The origins of bone marrow as the seedbed of our blood: from antiquity to the time of Osler». Baylor University Medical Center Proceedings. 24 (2): 115–8. doi:10.1080/08998280.2011.11928697. PMC 3069519. PMID 21566758.
- Wang J, Liu X, Lu H, Jiang C, Cui X, Yu L, Fu X, Li Q, Wang J (2015). «CXCR4(+)CD45(-) BMMNC subpopulation is superior to unfractionated BMMNCs for protection after ischemic stroke in mice». Brain Behav. Immun. 45: 98–108. doi:10.1016/j.bbi.2014.12.015. PMC 4342301. PMID 25526817.
External links[edit]
- Bone marrow histology photomicrographs