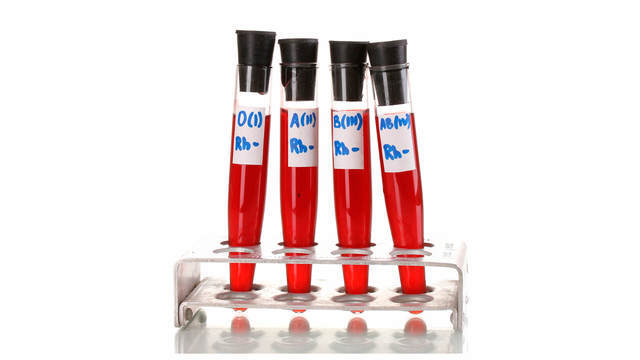
Всего столетие тому назад люди еще не имели такого подробного представления о составе кровяного русла и тем более, сколько групп крови существует, какое может сейчас получить любой интересующийся. Открытие всех групп крови принадлежит нобелевскому лауреату австрийскому ученому Карлу Ландштейнеру и его коллеге по исследовательской лаборатории. Группа крови как понятие стало употребляться с 1900 года. Разберемся, какие группы крови существуют и их характеристика.
Классификация по системе АВ0
Что такое группа крови? У каждого индивидуума в плазматической мембране эритроцитов есть около 300 различных антигенных элементов. Агглютиногенные частицы на молекулярном уровне по своей структуре закодированы посредством определенных форм одного и того же гена (аллеля) в одинаковых хромосомных участках (локусах).

Чем отличаются группы крови? Любая группа кровотока определяется специфическими системами антигенов эритроцитов, контролируемыми установленными локусами. И от того какие аллельные гены (обозначается буквами), в идентичных хромосомных участках находятся, и будет зависеть категория кровяной субстанции.
Точная численность локусов и аллелей к нынешнему моменту еще не имеет точных данных.
Какие бывают группы крови? Достоверно установлены около 50 разновидностей антигенов, но наиболее часто встречаются такие типы аллельных генов, как А и В. Поэтому именно они используются для обозначения групп плазмы. Особенности типа кровяной субстанции определяются объединением антигенных свойств кровотока, то есть унаследованных и переданных с кровью совокупностей генов. Каждое обозначение группы крови соответствует антигенным качествам красных кровяных телец, содержащихся в клеточной мембране.
Основная классификация групп крови по системе АВ0:
| Группы | Описание |
|---|---|
| I (0) | Отсутствие эритроцитарных антигенных свойств. |
| II (А) | Наличие в эритроцитарной оболочке антигена типа А. |
| III (В) | Присутствие в клеточной мембране эритроцитов антигена типа В. |
| IV (АВ) | Нахождение в плазматической оболочке красных кровяных телец антигенов обоих типов А и В. |
Виды групп крови различаются не только по категориям, есть еще такое понятие, как резус-фактор. Серологическая диагностика и обозначения группы крови и резус фактора всегда делаются одновременно. Потому как для переливания кровяной массы, например, жизненно важным значением является как группа кровяной субстанции, так и ее резус-фактор. И если группе крови свойственно иметь буквенное выражение, то резусные показатели всегда обозначались математическими символами такими как (+) и (−), что значит положительный или отрицательный резус-фактор.
Сочетаемость групп крови и резус-фактора
Резусной совместимости и по группам кровотока придается большое значение при переливании и планировании беременности, во избежание конфликтности эритроцитарной массы. Что касается переливания крови, особенно в экстренных ситуациях, эта процедура способна подарить пострадавшему жизнь. Только возможно это при идеальном совпадении всех компонентов крови. При малейшем несоответствии по группе либо резусу, может произойти склеивание эритроцитов, что влечет за собой, как правило, гемолитическую анемию или почечную недостаточность.
При таких обстоятельствах реципиента может постичь шоковое состояние, что нередко заканчивается летально.
Дабы исключить критические последствия гемотрансфузии, непосредственно перед вливанием крови медики проводят биологическую пробу на совместимость. Для этого реципиенту вливается небольшое количество цельной крови или отмытых эритроцитов и анализируется его самочувствие. Если отсутствуют симптомы, свидетельствующие о неприятии кровяной массы, то кровь можно вливать в полном, необходимом объеме.
Признаками отторжения кровяной жидкости (гемотрансфузионного шока) служат:
- озноб с выраженным ощущением холода;
- посинение кожи и слизистых;
- повышение температуры;
- появление судорог;
- тяжесть при дыхании, одышка;
- перевозбужденное состояние;
- снижение артериального давления;
- боли в поясничной области, в районе груди и живота, а также в мышцах.
Приведены наиболее характерные симптомы, которые возможны при вливании образца неподходящей кровяной субстанции. Внутрисосудистое введение кровяного вещества осуществляется под непрестанным контролем медицинского персонала, который при первых признаках шока должен приступить к реанимационным действиям в отношении реципиента. Гемотрансфузия требует высокого профессионализма, поэтому проводится строго в условиях стационара. Как влияют показатели кровяной жидкости на совместимость наглядно показано в таблице групп крови и резус-факторов.
Группы крови таблица:
| Группы крови обозначение и резус-фактор | Распространенность среди людей планеты | Для каких групп может быть донором | Какие категории кровотока подходят реципиенту |
|---|---|---|---|
| I (0) Rh «+» | 40–50% | 0, А, В, АВ с «+» и «−» | 0 с «+» и «−» |
| I (0) Rh «−» | 7–10% | 0, А, В, АВ с «−» | 0 с «−» |
| II (A) Rh «+» | 30–35% | А, АВ с «+» | 0, А с «+» и «−» |
| II (A) Rh «−» | 6–8% | А, АВ с «−» | 0, А с «−» |
| III (B) Rh «+» | 8–12% | В, АВ с «+» | 0, В с «+» и «−» |
| III (B) Rh «−» | 1–2% | В, АВ с «−» | 0, В с «−» |
| IV (AB) Rh «+» | 5–7% | АВ с «+» | 0, А, В, АВ с «+» |
| IV (AB) Rh «−» | менее 1% | АВ с «−» | АВ с «−» |
Схема, приведенная в таблице гипотетическая. На практике врачи отдают предпочтение классической гемотрансфузии ― это полное совпадение кровяной жидкости донора и реципиента. И лишь при крайней необходимости медицинский персонал решается на переливание допустимой крови.
Методы определения категорий крови
Диагностика на вычисление групп крови проводится после получения венозного или кровяного материала пациента. Чтобы установить резус-фактор понадобится кровь из вены, которую соединяют с двумя сыворотками (положительная и отрицательная).
О наличии у пациента того или иного резус-фактора свидетельствует образец, где нет агглютинации (склеивания эритроцитов).
Для определения группы кровяной массы используют следующие способы:
- Экспресс-диагностика применяется в экстренных случаях, ответ можно получить уже спустя три минуты. Осуществляется она с использованием пластиковых карточек с нанесенными на дно высушенными реактивами. Показывает одновременно группу и резус.
- Двойная перекрестная реакция используется для уточнения сомнительного результата исследования. Оценивают результат после смешивания сыворотки пациента с эритроцитарным материалом. Сведения доступны для интерпретации уже спустя 5 минут.
- Цоликлонирования при этом способе диагностики натуральные сыворотки подменяются искусственными цоликлонами (анти-А и -В).
- Стандартное определение категории кровотока выполняется путем соединения нескольких капель крови пациента с образцами сыворотки с четырьмя экземплярами известных антигенных фенотипов. Результат доступен в течение пяти минут.
Если агглютинация отсутствует во всех четырех образцах, то такой признак говорит, что перед вами первая группа. И в противоположность этому, когда во всех пробах происходит слипание эритроцитов, то этот факт указывает на четвертую группу. Касаемо второй и третьей категории крови, о каждой из них можно судить, в случае отсутствия агглютинации в биологическом образце сыворотки определяемой группы.
Отличительные свойства четырех групп крови
Характеристика групп крови позволяет судить не только о состоянии организма, физиологических особенностях и предпочтениях в пище. Вдобавок ко всем перечисленным сведениям, благодаря группам крови у человека, легко получить психологический портрет. Удивительно, но людьми давно подмечено, а учеными научно обосновано, что категории кровяной жидкости способны повлиять на личностные качества своих обладателей. Итак, рассмотрим описание группы крови и их характеристики.
Первая группа биологической среды человека принадлежит к самым истокам цивилизации и является самой многочисленной. Принято считать, что изначально 1 группа кровотока, свободная от агглютиногенных свойств эритроцитов, была у всех жителей Земли. Самые древние прародители выживали за счет охоты, ― это обстоятельство наложило свой отпечаток на их черты личности.
Психологический тип людей с «охотничьей» категорией крови:
- Целеустремленность.
- Лидерские качества.
- Уверенность в собственных силах.
К негативным аспектам личности относятся такие черты, как суетливость, ревность, чрезмерная амбициозность. Вполне естественно, что именно волевые качества характера и мощный инстинкт самосохранения способствовали выживанию предков и, тем самым сбережению расы доныне. Чтобы отлично себя чувствовать, представителям первого типа крови требуется преобладание белков в рационе и сбалансированное количество жиров и углеводов.
Формирование второй группы биологической жидкости начало происходить спустя примерно несколько десятков тысячелетий после первой. Состав крови стал претерпевать изменения из-за постепенного перехода многих общин на растительный вид питания, выращенный в процессе земледелия. Активное обрабатывание земли для культивирования различных злаков, плодовых и ягодных растений, привело к тому, что люди стали обосновываться в общины. Образ жизни в обществе и совместная трудовая занятость сказались как на изменении компонентов кровеносной системы, так и на личности индивидуумов.
Качества личности людей с «земледельческим» видом крови:
- Добросовестность и трудолюбие.
- Дисциплинированность, надежность, предусмотрительность.
- Доброжелательность, общительность и дипломатичность.
- Спокойный нрав и терпеливое отношение к окружающим.
- Организаторский талант.
- Быстрое приспособление к новой обстановке.
- Настойчивость в достижении намеченных целей.
В числе столь ценных качеств существовали и негативные черты характера, которые обозначим как чрезмерная осторожность и напряженность. Но это не перекрывает общего благоприятного впечатления от того, как на человечество повлияло разнообразие в питании и изменения в образе жизни. Особое внимание обладателям второй группы кровяного русла стоит уделить умению расслабляться. А насчет питания, то для них предпочтительна пища с преобладанием овощей, фруктов и злаков.
Мясо допускается белое лучше выбирать для питания легко усваиваемые белки.
Третья группа начала образовываться в результате волнообразного переселения жителей африканской местности на территории Европы, Америки, Азии. Особенности непривычного климата, другие продукты питания, развитие животноводства и прочие факторы стали причиной изменений, произошедших в кровеносной системе. Для людей этого типа крови, кроме мясных, полезны к тому же и молочные продукты животноводства. А также зерновые, бобовые, овощные, фруктовые и ягодные культуры.
Третья группа кровеносного русла говорит о своем владельце, что он:
- Выдающийся индивидуалист.
- Терпеливый и уравновешенный.
- Гибкий в партнерских отношениях.
- Сильный духом и оптимистично настроен.
- Слегка сумасбродный и непредсказуемый.
- Способный к оригинальному образу мыслей.
- Творческая личность с развитым воображением.
Среди такого количества полезных личностных качеств, неблагоприятно отличается только независимость «кочевников-скотоводов» и нежелание подчиняться сложившимся устоям. Хотя это почти не влияет на их взаимоотношения в обществе. Потому как эти люди, отличающиеся коммуникабельностью, легко найдут подход к любому человеку.
Особенности крови человека наложили свой отпечаток и на представителей земной расы с самой редкой группой кровяной субстанции ― четвертой.
Неординарная индивидуальность обладателей редчайшей четвертой категории крови:
- Творческое восприятие окружающего мира.
- Пристрастие ко всему прекрасному.
- Ярко выраженные интуитивные способности.
- Альтруисты по натуре, склонные к состраданию.
- Изысканный вкус.
В общем, носители четвертого типа крови отличаются уравновешенностью, чуткостью и врожденным чувством такта. Но иногда им свойственна резкость в высказываниях, что может создать неблагоприятное впечатление. Тонкая душевная организация и отсутствие напористости нередко вынуждают колебаться в принятии решения. Перечень разрешенных продуктов очень разнообразный, среди которого присутствуют продукты животного и растительного происхождения. Интересно отметить, что многие черты личности, которые люди приписывают обычно своим заслугам, оказываются всего лишь особенностями группы крови.
«Rh-» redirects here. For the Siddharta album, see Rh- (album). For the band, see The RH Factor. For the production company, see Regina Hall.
The Rh blood group system is a human blood group system. It contains proteins on the surface of red blood cells. After the ABO blood group system, it is the most likely to be involved in transfusion reactions. The Rh blood group system consists of 49 defined blood group antigens,[1] among which the five antigens D, C, c, E, and e are the most important. There is no d antigen. Rh(D) status of an individual is normally described with a positive (+) or negative (−) suffix after the ABO type (e.g., someone who is A+ has the A antigen and Rh(D) antigen, whereas someone who is A− has the A antigen but lacks the Rh(D) antigen). The terms Rh factor, Rh positive, and Rh negative refer to the Rh(D) antigen only. Antibodies to Rh antigens can be involved in hemolytic transfusion reactions and antibodies to the Rh(D) and Rh antigens confer significant risk of hemolytic disease of the fetus and newborn.
Nomenclature[edit]
| Fisher–Race | Wiener |
|---|---|
| Dce | R0 |
| DCe | R1 |
| DcE | R2 |
| DCE | RZ |
| dce | r |
| dCe | r’ |
| dcE | r″ |
| dCE | ry |
The Rh blood group system has two sets of nomenclatures: one developed by Ronald Fisher and R. R. Race, the other by Wiener. Both systems reflected alternative theories of inheritance. The Fisher–Race system, which is more commonly in use today, uses the CDE nomenclature. This system was based on the theory that a separate gene controls the product of each corresponding antigen (e.g., a «D gene» produces D antigen, and so on). However, the d gene was hypothetical, not actual.
The Wiener system used the Rh–Hr nomenclature. This system was based on the theory that there was one gene at a single locus on each of the 2 copies of chromosome 1, each contributing to production of multiple antigens. In this theory, a gene R1 is supposed to give rise to the «blood factors» Rh0, rh′, and rh″ (corresponding to modern nomenclature of the D, C, and E antigens) and the gene r to produce hr′ and hr″ (corresponding to modern nomenclature of the c and e antigens).[3]
Notations of the two theories are used interchangeably in blood banking (e.g., Rho(D) meaning RhD positive). Wiener’s notation is more complex and cumbersome for routine use. Because it is simpler to explain, the Fisher–Race theory has become more widely used.[citation needed]
DNA testing has shown that both are partially correct: There are in fact two linked genes, the RHD gene which produces a single immune specificity (anti-D) and the RHCE gene with multiple specificities (anti-C, anti-c, anti-E, anti-e). Thus, Wiener’s postulate that a gene could have multiple specificities (something many did not give credence to originally) has been proved to be correct. On the other hand, Wiener’s theory that there is only one gene has proved to be incorrect, as has the Fisher–Race theory that there are three genes, rather than the 2. The CDE notation used in the Fisher–Race nomenclature is sometimes rearranged to DCE to more accurately represent the co-location of the C and E encoding on the RhCE gene, and to make interpretation easier.[citation needed]
Antigens[edit]
The proteins which carry the Rh antigens are transmembrane proteins, whose structure suggest that they are ion channels.[4] The main antigens are D, C, E, c and e, which are encoded by two adjacent gene loci, the RHD gene which encodes the RhD protein with the D antigen (and variants)[5] and the RHCE gene which encodes the RhCE protein with the C, E, c and e antigens (and variants).[6] There is no d antigen. Lowercase «d» indicates the absence of the D antigen (the gene is usually deleted or otherwise nonfunctional).[citation needed]
1. This is the Rh-positive blood cell.
2. This is the Rh-negative blood cell.
3. These are the antigens on the Rh-positive blood cell that make it positive. The antigens allow the positive blood cell to attach to specific antibodies.
Rh phenotypes are readily identified through the presence or absence of the Rh surface antigens. As can be seen in the table below, most of the Rh phenotypes can be produced by several different Rh genotypes. The exact genotype of any individual can only be identified by DNA analysis. Regarding patient treatment, only the phenotype is usually of any clinical significance to ensure a patient is not exposed to an antigen they are likely to develop antibodies against. A probable genotype may be speculated on, based upon the statistical distributions of genotypes in the patient’s place of origin.[citation needed]
R0 (cDe or Dce) is today most common in Africa. The allele was thus often assumed in early blood group analyses to have been typical of populations on the continent; particularly in areas below the Sahara. Ottensooser et al. (1963) suggested that high R0 frequencies were likely characteristic of the ancient Judea Jews, who had emigrated from Egypt prior to their dispersal throughout the Mediterranean Basin and Europe[7] on the basis of high R0 percentages among Sephardi and Ashkenazi Jews compared to native European populations and the relative genetic isolation of Ashkenazim. However, more recent studies have found R0 frequencies as low as 24.3% among some Afroasiatic-speaking groups in the Horn of Africa,[8] as well as higher R0 frequencies among certain other Afroasiatic speakers in North Africa (37.3%)[9] and among some Palestinians in the Levant (30.4%).[10] On the contrary, at a frequency of 47.2% of the population of Basque country having the lack of the D antigen, these people display the highest frequency of the Rh negative phenotype.[11]
| Phenotype expressed on cell | Genotype expressed in DNA | Prevalence (%) |
|
|---|---|---|---|
| Fisher–Race notation | Wiener notation | ||
| D+ C+ E+ c+ e+ (RhD+) | Dce/DCE | R0RZ | 0.0125 |
| Dce/dCE | R0rY | 0.0003 | |
| DCe/DcE | R1R2 | 11.8648 | |
| DCe/dcE | R1r″ | 0.9992 | |
| DcE/dCe | R2r′ | 0.2775 | |
| DCE/dce | RZr | 0.1893 | |
| D+ C+ E+ c+ e− (RhD+) | DcE/DCE | R2RZ | 0.0687 |
| DcE/dCE | R2rY | 0.0014 | |
| DCE/dcE | RZr″ | 0.0058 | |
| D+ C+ E+ c− e+ (RhD+) | DCe/dCE | R1rY | 0.0042 |
| DCE/dCe | RZr′ | 0.0048 | |
| DCe/DCE | R1RZ | 0.2048 | |
| D+ C+ E+ c− e− (RhD+) | DCE/DCE | RZRZ | 0.0006 |
| DCE/dCE | RZrY | < 0.0001 | |
| D+ C+ E− c+ e+ (RhD+) | Dce/dCe | R0r′ | 0.0505 |
| DCe/dce | R1r | 32.6808 | |
| DCe/Dce | R1R0 | 2.1586 | |
| D+ C+ E− c− e+ (RhD+) | DCe/DCe | R1R1 | 17.6803 |
| DCe/dCe | R1r′ | 0.8270 | |
| D+ C− E+ c+ e+ (RhD+) | DcE/Dce | R2R0 | 0.7243 |
| Dce/dcE | R0r″ | 0.0610 | |
| DcE/dce | R2r | 10.9657 | |
| D+ C− E+ c+ e− (RhD+) | DcE/DcE | R2R2 | 1.9906 |
| DcE/dcE | R2r″ | 0.3353 | |
| D+ C− E− c+ e+ (RhD+) | Dce/Dce | R0R0 | 0.0659 |
| Dce/dce | R0r | 1.9950 | |
| D− C+ E+ c+ e+ (RhD−) | dce/dCE | rrY | 0.0039 |
| dCe/dcE | r′r″ | 0.0234 | |
| D− C+ E+ c+ e− (RhD−) | dcE/dCE | r″rY | 0.0001 |
| D− C+ E+ c− e+ (RhD−) | dCe/dCE | r′rY | 0.0001 |
| D− C+ E+ c− e− (RhD−) | dCE/dCE | rYrY | < 0.0001 |
| D− C+ E− c+ e+ (RhD−) | dce/dCe | rr′ | 0.7644 |
| D− C+ E− c− e+ (RhD−) | dCe/dCe | r′r′ | 0.0097 |
| D− C− E+ c+ e+ (RhD−) | dce/dcE | rr″ | 0.9235 |
| D− C− E+ c+ e− (RhD−) | dcE/dcE | r″r″ | 0.0141 |
| D− C− E− c+ e+ (RhD−) | dce/dce | rr | 15.1020 |
• Figures taken from a study performed in 1948 on a sample of 2000 people in the United Kingdom.[12]
| Rh Phenotype | CDE | Patients (%) | Donors (%) |
|---|---|---|---|
| R1r | CcDe | 37.4 | 33.0 |
| R1R2 | CcDEe | 35.7 | 30.5 |
| R1R1 | CDe | 5.7 | 21.8 |
| rr | ce | 10.3 | 11.6 |
| R2r | cDEe | 6.6 | 10.4 |
| R0R0 | cDe | 2.8 | 2.7 |
| R2R2 | cDE | 2.8 | 2.4 |
| rr″ | cEe | – | 0.98 |
| RZRZ | CDE | – | 0.03 |
| rr′ | Cce | 0.8 | – |
Rh antibodies[edit]
Rh antibodies are Immunoglobulin G (IgG) antibodies which are acquired through exposure to Rh-positive blood (generally either through pregnancy or transfusion of blood products). The D antigen is the most immunogenic of all the non-ABO antigens. Approximately 80% of individuals who are D-negative and exposed to a single D-positive unit will produce an anti-D antibody. The percentage of alloimmunization is significantly reduced in patients who are actively exsanguinating.[14]
All Rh antibodies except D display dosage (antibody reacts more strongly with red cells homozygous for an antigen than cells heterozygous for the antigen (EE stronger reaction vs Ee).
If anti-E is detected, the presence of anti-c should be strongly suspected (due to combined genetic inheritance). It is therefore common to select c-negative and E-negative blood for transfusion patients who have an anti-E and lack the c antigen (in general, a patient will not produce antibodies against their own antigens). Anti-c is a common cause of delayed hemolytic transfusion reactions.[15]
Hemolytic disease of the newborn[edit]
The hemolytic condition occurs when there is an incompatibility between the blood types of the mother and fetus. There is also potential incompatibility if the mother is Rh negative and the father is positive. When any incompatibility is detected, the mother often receives an injection at 28 weeks gestation and at birth to avoid the development of antibodies towards the fetus. These terms do not indicate which specific antigen-antibody incompatibility is implicated. The disorder in the fetus due to Rh D incompatibility is known as erythroblastosis fetalis.
- Hemolytic comes from two words: «hema» (blood) and «lysis» (solution) or breaking down of red blood cells
- Erythroblastosis refers to the making of immature red blood cells
- Fetalis refers to the fetus.
When the condition is caused by the Rh D antigen-antibody incompatibility, it is called Rh D Hemolytic disease of the newborn or Rh disease. Here, sensitization to Rh D antigens (usually by feto-maternal transfusion during pregnancy) may lead to the production of maternal IgG anti-D antibodies which can pass through the placenta. This is of particular importance to D negative females at or below childbearing age, because any subsequent pregnancy may be affected by the Rh D hemolytic disease of the newborn if the baby is D positive. The vast majority of Rh disease is preventable in modern antenatal care by injections of IgG anti-D antibodies (Rho(D) Immune Globulin). The incidence of Rh disease is mathematically related to the frequency of D negative individuals in a population, so Rh disease is rare in old-stock populations of Africa and the eastern half of Asia, and the Indigenous peoples of Oceania and the Americas, but more common in other genetic groups, most especially Western Europeans, but also other West Eurasians, and to a lesser degree, native Siberians, as well as those of mixed-race with a significant or dominant descent from those (e.g. the vast majority of Latin Americans and Central Asians).
- Symptoms and signs in the fetus:
- Enlarged liver, spleen, or heart and fluid buildup in the fetus’ abdomen seen via ultrasound.
- Symptoms and signs in the newborn:
- Anemia that creates the newborn’s pallor (pale appearance).
- Jaundice or yellow discoloration of the newborn’s skin, sclera or mucous membrane. This may be evident right after birth or after 24–48 hours after birth. This is caused by bilirubin (one of the end products of red blood cell destruction).
- Enlargement of the newborn’s liver and spleen.
- The newborn may have severe edema of the entire body.
- Dyspnea (difficulty breathing)
Other animals with Rh-like antigens causing hemolytic disease of the newborn
Rh disease only occurs in human fetuses however a similar disease called Neonatal isoerythrolysis (NI) can be observed in animal species of newborn horses, mules, pigs, cats, cattle, and dogs. What differs between Rh disease and NI is the pathogenesis of hemolysis between human fetuses and the animal species. With human mothers, the maternal antibodies are formed from the sensitization of foreign antigens of her unborn fetus’s red blood cells passing through the placenta causing hemolysis before birth, with other animals however, these maternal antibodies are not passed through the placenta but through colostrum. The newborn animal is without NI but soon develops hemolytic anemia after initial ingestion of its mother’s colostrum that contain antibodies that can be absorbed through the newborn’s intestines and are incompatible to its red blood cell antigen. After 48 hours of birth, the newborn may be allowed to nurse from its mother as her antibodies can no longer be absorbed through the neonate’s intestines. Because the most active newborn animals consume the most colostrum, they may be the ones who are most affected by the blood incompatibility of antigen and antibody.[16]
Rh proteins outside of human species
Rh molecules can be found in many different living organisms from worms, bacteria, algae, and other vertebrates. These Rh molecules from different animals have the same biochemical function-differing slightly in their amino acid sequences.[17] The Rh proteins in other species, however, do not correspond with the Rh blood group or antigens found on human red blood cells. One such example would be the nematode Caenorhabditis elegans. Because this worm does not have red blood cells, it cannot have Rh antigens, excluding it from having a Rh blood type. These Rh proteins therefore do not bind to red blood cells; they operate independently. Instead of transporting CO2 from the proteins of human red blood cells, C. elegan’s Rh proteins transport NH3 out of its body.[18]
Population data[edit]
According to a comprehensive study, the worldwide frequency of Rh-positive and Rh-negative blood types is approximately 94% and 6%, respectively. The same study concluded that the share of the population with Rh-negative blood type is set to fall further in the future primarily due to low population growth in Europe.[19] The frequency of Rh factor blood types and the RhD neg allele gene differs in various populations.[citation needed]
| Population | Rh(D) Neg | Rh(D) Pos | Rh(D) Neg alleles |
|---|---|---|---|
| African Americans | ∼ 7% | 93% | ∼ 26% |
| Albania[21] | 10.86% | 89% | weak D 1.4% |
| Basques[22] | 21%–36% | 65% | ∼ 60% |
| Britain[23] | 17% | 83% | |
| China[23] | < 1% | > 99% | |
| Ethiopians[23] | 1%–21% | 99%–79% | |
| Europeans (others) | 16% | 84% | 40% |
| India[23] | 0.6%–8.4% | 99.4%–91.6% | |
| Indonesia[23] | < 1% | > 99% | |
| Japan[23] | < 1% | > 99% | |
| Koreans[24] | < 1% | > 99% | |
| Madagascar[23] | 1% | 99% | |
| Moroccans[25] | 9.5% | 90.5% | |
| Moroccans (High Atlas)[26] | ∼ 29% | 71% | |
| Native Americans | ∼ 1% | 99% | ∼ 10% |
| Nigeria[27] | 6% | 94% | |
| Saudi Arabia[28] | 8.8% | 91.2% | 29.5% |
| Subequatorial Africa[23] | 1%–3% | 99%–97% | |
| United States[23] | 15% | 85% |
Genetics[edit]
This is a Punnett square for Rh factor inheritance. This square specifically shows two heterozygous Rh positive parents and the possible genotypes/phenotypes the offspring could have.
The D antigen is inherited as one gene (RHD) (on the short arm of the first chromosome, p36.13–p34.3) with various alleles. Typically, Rhesus positive people have an intact RHD gene while negative people lack the gene (or have mutations in it). However, there are exceptions: for instance, Japanese and black Africans may have an intact gene that is not expressed or only at very low levels.[29] The gene codes for the RhD protein on the red blood cell membrane. D− individuals who lack a functional RHD gene do not produce the D antigen, and may be immunized by D+ blood.[citation needed]
The D antigen is a dominant trait. If both of a child’s parents are Rh negative, the child will definitely be Rh negative. Otherwise the child may be Rh positive or Rh negative, depending on the parents’ specific genotypes.[30]
The epitopes for the next 4 most common Rh antigens, C, c, E and e are expressed on the highly similar RhCE protein that is genetically encoded in the RHCE gene, also found on chromosome 1. It has been shown that the RHD gene arose by duplication of the RHCE gene during primate evolution. Mice have just one RH gene.[31]
The RHAG gene, which is responsible for encoding Rh-associated glycoprotein (RhAG), is found on chromosome 6a.
The polypeptides produced from the RHD and RHCE genes form a complex on the red blood cell membrane with the Rh-associated glycoprotein.[15]
Function[edit]
| Blood group Rh C/E/D polypeptide | |
|---|---|
| Identifiers | |
| Symbol | ? |
| InterPro | IPR002229 |
On the basis of structural homology it has been proposed that the product of RHD gene, the RhD protein, is a membrane transport protein of uncertain specificity (CO2 or NH3) and unknown physiological role.[32][33] The three-dimensional structure of the related RHCG protein and biochemical analysis of the RhD protein complex indicates that the RhD protein is one of three subunits of an ammonia transporter.[34][35] Three recent studies[36][37][38] have reported a protective effect of the RhD-positive phenotype, especially RhD heterozygosity, against the negative effect of latent toxoplasmosis on psychomotor performance in infected subjects. RhD-negative compared to RhD-positive subjects without anamnestic titres of anti-Toxoplasma antibodies have shorter reaction times in tests of simple reaction times. And conversely, RhD-negative subjects with anamnestic titres (i.e. with latent toxoplasmosis) exhibited much longer reaction times than their RhD-positive counterparts. The published data suggested that only the protection of RhD-positive heterozygotes was long term in nature; the protection of RhD-positive homozygotes decreased with duration of the infection while the performance of RhD-negative homozygotes decreased immediately after the infection. The overall change in reaction times was always larger in the RhD-negative group than in the RhD-positive.[citation needed]
RHD polymorphism[edit]
Origin of RHD polymorphism[edit]
For a long time, the origin of RHD polymorphism was an evolutionary enigma.[39][40][41] Before the advent of modern medicine, the carriers of the rarer allele (e.g. RhD-negative women in a population of RhD positives or RhD-positive men in a population of RhD negatives) were at a disadvantage as some of their children (RhD-positive children born to preimmunised RhD-negative mothers) were at a higher risk of fetal or newborn death or health impairment from hemolytic disease.[citation needed]
Natural selection aside, the RHD-RHCE region is structurally predisposed to many mutations seen in humans, since the pair arose by gene duplication and remain similar enough for unequal crossing over to occur.[31] In addition to the case where D is deleted, crossover can also produce a single gene mixing exons from both RHD and RHCE, forming the majority of partial D types.[42]: 323
Weak D[edit]
| Weak D | Partial D | |
|---|---|---|
| Change in D | Decreased amount | Structural alternation |
| Can donate as if being: |
D positive | D positive |
| Can receive blood as if being: |
D positive (usually)[42] | D negative[15] |
In serologic testing, D positive blood is easily identified. Units that are D negative are often retested to rule out a weaker reaction. This was previously referred to as Du, which has been replaced.[42]: 322 By definition, weak D phenotype is characterized by negative reaction with anti-D reagent at immediate spin (IS), negative reaction after 37 °C incubation, and positive reaction at anti-human globulin (AHG) phase. Weak D phenotype can occur in several ways. In some cases, this phenotype occurs because of an altered surface protein that is more common in people of European descent. An inheritable form also occurs, as a result of a weakened form of the R0 gene. Weak D may also occur as «C in trans», whereby a C gene is present on the opposite chromosome to a D gene (as in the combination R0r’, or «Dce/dCe»). The testing is difficult, since using different anti-D reagents, especially the older polyclonal reagents, may give different results.
The practical implication of this is that people with this sub-phenotype will have a product labeled as «D positive» when donating blood. When receiving blood, they are sometimes typed as a «D negative», though this is the subject of some debate. Most «Weak D» patients can receive «D positive» blood without complications.[42]: 323 However, it is important to correctly identify the ones that have to be considered D+ or D−. This is important, since most blood banks have a limited supply of «D negative» blood and the correct transfusion is clinically relevant. In this respect, genotyping of blood groups has much simplified this detection of the various variants in the Rh blood group system.
Partial D[edit]
It is important to differentiate weak D (due to a quantitative difference in the D antigen) from partial D (due to a qualitative difference in the D antigen). Simply put, the weak D phenotype is due to a reduced number of D antigens on a red blood cell. In contrast, the partial D phenotype is due to an alteration in D-epitopes. Thus, in partial D, the number of D antigens is not reduced but the protein structure is altered. These individuals, if alloimmunized to D, can produce an anti-D antibody. Therefore, partial D patients who are donating blood should be labeled as D-positive but, if receiving blood, they should be labeled as D-negative and receive D-negative units.[15]
In the past, partial D was called ‘D mosaic’ or ‘D variant.’ Different partial D phenotypes are defined by different D epitopes on the outer surface of the red blood cell membrane. More than 30 different partial D phenotypes have been described.[15]
Rhnull phenotype[edit]
Rhnull individuals have no Rh antigens (no Rh or RhAG) on their red blood cells.[43] This rare condition[43] has been called «Golden Blood».[44] As a consequence of Rh antigen absence, Rhnull red blood cells also lack LW and Fy5 and show weak expression of S, s, and U antigens.
Red blood cells lacking Rh/RhAG proteins have structural abnormalities (such as stomatocytosis) and cell membrane defects that can result in hemolytic anemia.[15][43]
The first Rhnull blood was discovered in an Aboriginal Australian woman, in 1961.[45] Only 43 individuals have been reported to have it worldwide. Only nine active donors have been reported.[44] Its properties make it attractive in numerous medical applications, but scarcity makes it expensive to transport and acquire.[46]
Other Rh group antigens[edit]
Currently, 50 antigens have been described in the Rh group system; among those described here, the D, C, c, E and e antigens are the most important. The others are much less frequently encountered or are rarely clinically significant. Each is given a number, though the highest assigned number (CEVF or RH61 according to the ISBT terminology) is not an accurate reflection of the antigens encountered since many (e.g. Rh38) have been combined, reassigned to other groups, or otherwise removed.[42]: 324
Some of the other Rh «antigens» are f («ce», RH6), Ce (RH7), Cw (RH8), Cx (RH9), V (RH10), Ew (RH11), G (RH12), Tar (RH40), VS (RH20), Dw (RH23), and CE (RH22). Some of these groups, including f, Ce and CE, describe grouping of some existing groups. Others, like V, describe an epitope created by some other mutation on the RHD and RHCE genes. V in particular is caused by a mutation on RHCE.[47]
History[edit]
The term «Rh» was originally an abbreviation of «Rhesus factor». It was discovered in 1937 by Karl Landsteiner and Alexander S. Wiener, who, at the time, believed it to be a similar antigen found in rhesus macaque red blood cells. It was subsequently discovered that the human factor is not identical to the rhesus monkey factor, but by then, «Rhesus Group» and like terms were already in widespread, worldwide use. Thus, notwithstanding it is a misnomer, the term survives (e.g., rhesus blood group system and the obsolete terms rhesus factor, rhesus positive, and rhesus negative – all three of which actually refer specifically and only to the Rh D factor and are thus misleading when unmodified). Contemporary practice is to use «Rh» as a term of art instead of «Rhesus» (e.g., «Rh Group», «Rh factors», «Rh D», etc.).
The significance of their discovery was not immediately apparent and was only realized in 1940, after subsequent findings by Philip Levine and Rufus Stetson.[48] The serum that led to the discovery was produced by immunizing rabbits with red blood cells from a rhesus macaque. The antigen that induced this immunization was designated by them as Rh factor to indicate that rhesus blood had been used for the production of the serum.[49]
In 1939, Phillip Levine and Rufus Stetson published in a first case report the clinical consequences of non-recognized Rh factor, hemolytic transfusion reaction, and hemolytic disease of the newborn in its most severe form.[50] It was recognized that the serum of the reported woman agglutinated with red blood cells of about 80% of the people although the then known blood groups, in particular ABO were matched. No name was given to this agglutinin when described. In 1940, Karl Landsteiner and Alexander S. Wiener made the connection to their earlier discovery, reporting a serum that also reacted with about 85% of different human red blood cells.[51]
In 1941, Group O: a patient of Dr. Paul in Irvington, NJ, delivered a normal infant in 1931; this pregnancy was followed by a long period of sterility. The second pregnancy (April, 1941) resulted in an infant with icterus gravis.[52] In May 1941, the third anti-Rh serum (M.S.) of Group O became available.[52]
Based on the serologic similarities, Rh factor was later also used for antigens, and anti-Rh for antibodies, found in humans such as those previously described by Levine and Stetson. Although differences between these two sera were shown already in 1942 and clearly demonstrated in 1963, the already widely used term «Rh» was kept for the clinically described human antibodies which are different from the ones related to the rhesus monkey. This real factor found in rhesus macaque was classified in the Landsteiner-Weiner antigen system (antigen LW, antibody anti-LW) in honor of the discoverers.[53][54]
It was recognized that the Rh factor was just one in a system of various antigens. Based on different models of genetic inheritance, two different terminologies were developed; both of them are still in use.
The clinical significance of this highly immunizing D antigen (i.e., Rh factor) was soon realized. Some keystones were to recognize its importance for blood transfusion (including reliable diagnostic tests), hemolytic disease of the newborn (including exchange transfusion), and very importantly the prevention of it by screening and prophylaxis.
The discovery of fetal cell-free DNA in maternal circulation by Holzgrieve et al. led to the noninvasive genotyping of fetal Rh genes in many countries.
References[edit]
- ^ Dean, Laura. Blood Groups and Red Cell Antigens [Internet].. Bethesda (MD): National Center for Biotechnology Information (US); 2005, Chapter. 7.
- ^ «Rh System». Canadian Blood Services at learnserology.ca. Retrieved 2021-01-19.
- ^ Weiner AS (1 February 1949). «Genetics and Nomenclature of the Rh–Hr Blood Types». Antonie van Leeuwenhoek. 15 (1): 17–28. doi:10.1007/BF02062626. ISSN 0003-6072. S2CID 35680084.
- ^ «dbRBC – Blood Group Antigen Gene Mutation Database». www.ncbi.nlm.nih.gov. Archived from the original on 2011-02-13. Retrieved 2010-06-15.
- ^ «RHD Rh blood group, D antigen [Homo sapiens] – Gene Result». nlm.nih.gov. Retrieved 2010-06-15.
- ^ «RHCE Rh blood group, CcEe antigens [Homo sapiens] – Gene Result». nlm.nih.gov. Archived from the original on 2010-03-20. Retrieved 2010-06-15.
- ^ Ottensooser F, Leon N, Sato M, Saldanha PH (March 1963). «Blood groups of a population of Ashkenazi Jews in Brazil». American Journal of Physical Anthropology. 21 (1): 41–8. doi:10.1002/ajpa.1330210106. PMID 13940710. S2CID 21795826.
- ^ Harrison GA, Küchemann CF, Moore MA, Boyce AJ, Baju T, Mourant AE, et al. (1969). «The effects of altitudinal variation in Ethiopian populations». Philosophical Transactions of the Royal Society of London B: Biological Sciences. 256 (805): 147–182. Bibcode:1969RSPTB.256..147H. doi:10.1098/rstb.1969.0040.
- ^ Metri AA, Sidi-Yakhlef A, Biémont C, Saidi M, Chaif O, Ouraghi SA (2012). «A genetic study of nine populations from the region of Tlemcen in Western Algeria: a comparative analysis on the Mediterranean scale». Anthropological Science. 120 (3): 209–216. doi:10.1537/ase.120618. Archived from the original on 29 August 2017. Retrieved 28 August 2017.
- ^ El-Wahhab Skaik YA (July 2011). «The Rh allele frequencies in Gaza city in Palestine». Asian Journal of Transfusion Science. 5 (2): 150–2. doi:10.4103/0973-6247.83241. PMC 3159245. PMID 21897594.
- ^ Flores-Bello A, Mas-Ponte D, Rosu ME, Bosch E, Calafell F, Comas D (December 2018). «Sequence diversity of the Rh blood group system in Basques». European Journal of Human Genetics. 26 (12): 1859–1866. doi:10.1038/s41431-018-0232-1. PMC 6244411. PMID 30089826.
- ^ Race RR, Mourant AE (June 1948). «The Rh chromosome frequencies in England». Blood. 3 (6): 689–95. doi:10.1182/blood.V3.6.689.689. PMID 18860341.
- ^ Canatan D, Acar N, Kiliç B (1999). «Rh Subgroups and Kell Antigens in Patients With Thalassemia and in Donors in Turkey» (PDF). Turkish Journal of Medical Sciences. 29: 155–7. Archived from the original (PDF) on 2008-12-17. Retrieved 2008-10-17.
- ^ Roback et al. AABB Technical Manual, 16th Ed. Bethesda, AABB Press, 2008.
- ^ a b c d e f Mais, DD. ASCP Quick Compendium of Clinical Pathology, 2nd Ed. Chicago, ASCP Press, 2009.
- ^ Cotter S (June 1, 2001). Hematology. Quick Look Series (1st ed.). Teton NewMedia. pp. 32–33. ISBN 978-1893441361.
- ^ Ji Q, Hashmi S, Liu Z, Zhang J, Chen Y, Huang CH (April 2006). «CeRh1 (rhr-1) is a dominant Rhesus gene essential for embryonic development and hypodermal function in Caenorhabditis elegans». Proceedings of the National Academy of Sciences of the United States of America. 103 (15): 5881–5886. doi:10.1073/pnas.0600901103. PMC 1458667. PMID 16595629.
- ^ «What is Rhesus factor? Did we get it from Rhesus monkeys? Can Rh factor be found in other animals?». The Tech Interactive. 2018-11-20. Retrieved 2022-10-24.
- ^ «Blood Type Frequencies by Country including the Rh Factor – Rhesus Negative».
- ^ Mack S (March 21, 2001). «Re: Is the RH negative blood type more prevalent in certain ethnic groups?». MadSci Network. Archived from the original on February 24, 2011.
- ^ Xhetani M, Seferi I, Férec C, Zoraqi G, Fichou Y (October 2014). «Distribution of Rhesus blood group antigens and weak D alleles in the population of Albania». Blood Transfusion = Trasfusione del Sangue. 12 (4): 565–9. doi:10.2450/2014.0240-13. PMC 4212038. PMID 24960662.
- ^ Touinssi M, Chiaroni J, Degioanni A, De Micco P, Dutour O, Bauduer F (2004). «Distribution of rhesus blood group system in the French basques: a reappraisal using the allele-specific primers PCR method». Human Heredity. 58 (2): 69–72. doi:10.1159/000083027. PMID 15711086. S2CID 44542508.
- ^ a b c d e f g h i Golassa L, Tsegaye A, Erko B, Mamo H (July 2017). «High rhesus (Rh(D)) negative frequency and ethnic-group based ABO blood group distribution in Ethiopia». BMC Research Notes. 10 (1): 330. doi:10.1186/s13104-017-2644-3. PMC 5530478. PMID 28747227.
- ^ Kim JY, Kim SY, Kim CA, Yon GS, Park SS (March 2005). «Molecular characterization of D- Korean persons: development of a diagnostic strategy». Transfusion. 45 (3): 345–52. doi:10.1111/j.1537-2995.2005.04311.x. PMID 15752151. S2CID 42899178.
- ^ Wafi ME, Housse HE, Nourichafi N, Bouisk K, Benajiba M, Habti N (2016). «Prevalence of weak D phenotype among D negative C/E+ blood donors in Morocco» (PDF). International Journal of Blood Transfusion and Immunohematology. 6 (1): 3–7. doi:10.5348/ijbti-2016-22-OA-2. Archived (PDF) from the original on August 28, 2016. Retrieved February 3, 2018.
- ^ Weinstock C (January 2014). «It is worthwhile filling in the remaining blank spots for blood group antigen frequencies». Blood Transfusion = Trasfusione del Sangue. 12 (1): 3–6. doi:10.2450/2013.0192-13. PMC 3926725. PMID 24120599.
- ^ Enosolease ME, Bazuaye GN (January 2008). «Distribution of ABO and Rh-D blood groups in the Benin area of Niger-Delta: Implication for regional blood transfusion». Asian Journal of Transfusion Science. 2 (1): 3–5. doi:10.4103/0973-6247.39502. PMC 2798756. PMID 20041069.
- ^ Eweidah MH, Rahiman S, Ali MH, Al-Shamary AM (April 2011). «Distribution of ABO and Rhesus (RHD) Blood Groups in Al-Jouf Province of the Saudi Arabia» (PDF). The Anthropologist. 13 (2): 99–102. doi:10.1080/09720073.2011.11891182. S2CID 75537061. Archived (PDF) from the original on January 2, 2013. Retrieved February 3, 2018.
- ^ Avent ND, Reid ME (January 2000). «The Rh blood group system: a review». Blood. 95 (2): 375–387. doi:10.1182/blood.V95.2.375. PMID 10627438. S2CID 13803474.
- ^ «ABO inheritance patterns». Inheritance patterns of blood groups. Australian Red Cross Blood Service. Archived from the original on 1 November 2013. Retrieved 30 October 2013.
- ^ a b Wagner FF, Flegel WA (March 2002). «RHCE represents the ancestral RH position, while RHD is the duplicated gene». Blood. 99 (6): 2272–3. doi:10.1182/blood-2001-12-0153. PMID 11902138.
- ^ Kustu S, Inwood W (2006). «Biological gas channels for NH3 and CO2: evidence that Rh (Rhesus) proteins are CO2 channels». Transfusion Clinique et Biologique. 13 (1–2): 103–10. doi:10.1016/j.tracli.2006.03.001. PMID 16563833.
- ^ Biver S, Scohy S, Szpirer J, Szpirer C, André B, Marini AM (2006). «Physiological role of the putative ammonium transporter RhCG in the mouse». Transfusion Clinique et Biologique. 13 (1–2): 167–8. doi:10.1016/j.tracli.2006.03.003. PMID 16564721.
- ^ Gruswitz F, Chaudhary S, Ho JD, Schlessinger A, Pezeshki B, Ho CM, et al. (May 2010). «Function of human Rh based on structure of RhCG at 2.1 A». Proceedings of the National Academy of Sciences of the United States of America. 107 (21): 9638–43. Bibcode:2010PNAS..107.9638G. doi:10.1073/pnas.1003587107. PMC 2906887. PMID 20457942.
- ^ Westhoff CM (January 2007). «The structure and function of the Rh antigen complex». Seminars in Hematology. 44 (1): 42–50. doi:10.1053/j.seminhematol.2006.09.010. PMC 1831834. PMID 17198846.
- ^ Novotná M, Havlícek J, Smith AP, Kolbeková P, Skallová A, Klose J, et al. (September 2008). «Toxoplasma and reaction time: role of toxoplasmosis in the origin, preservation and geographical distribution of Rh blood group polymorphism». Parasitology. 135 (11): 1253–61. doi:10.1017/S003118200800485X. PMID 18752708. S2CID 5956901.
- ^ Flegr J, Novotná M, Lindová J, Havlícek J (August 2008). «Neurophysiological effect of the Rh factor. Protective role of the RhD molecule against Toxoplasma-induced impairment of reaction times in women» (PDF). Neuro Endocrinology Letters. 29 (4): 475–81. PMID 18766148.
- ^ Flegr J, Klose J, Novotná M, Berenreitterová M, Havlícek J (May 2009). «Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study». BMC Infectious Diseases. 9: 72. doi:10.1186/1471-2334-9-72. PMC 2692860. PMID 19470165.
- ^ Haldane JB (1942). «Selection against heterozygosis in Man». Annals of Eugenics. 11: 333–340. doi:10.1111/j.1469-1809.1941.tb02297.x.
- ^ Fisher RA, Race RR, Taylor GL (1944). «Mutation and the Rhesus reaction». Nature. 153 (3873): 106. Bibcode:1944Natur.153..106F. doi:10.1038/153106b0. S2CID 2104065.
- ^ Li CC (1953). «Is the Rh facing a crossroad? A critique of the compensation effect». Am Nat. 87 (835): 257–261. doi:10.1086/281782. S2CID 84715943.
- ^ a b c d e Brecher ME (2005). Technical Manual (15th ed.). Bethesda MD: American Association of Blood Banks. ISBN 978-1-56395-196-1.
- ^ a b c Cartron JP (December 1999). «RH blood group system and molecular basis of Rh-deficiency». Bailliere’s Best Practice & Research. Clinical Haematology. 12 (4): 655–89. doi:10.1053/beha.1999.0047. PMID 10895258.
- ^ a b «Rhnull, the Rarest Blood Type on Earth, Has Been Called the «Golden Blood»«. Curiosity.com. Archived from the original on 5 December 2019. Retrieved 2019-06-05.
- ^ «J-STAGE».
- ^ Bailey P. «The man with the golden blood». Mosaic Science. Retrieved 16 January 2019.
- ^ «Rh System: Anti-V». Professional Education. 2 October 2019. Archived from the original on 7 November 2020.
- ^ Landsteiner K, Wiener AS (1940). «An Agglutinable Factor in Human Blood Recognized by Immune Sera for Rhesus Blood». Exp Biol Med (Maywood). 43 (1): 223. doi:10.3181/00379727-43-11151. S2CID 58298368.
- ^ Landsteiner K, Wiener AS (September 1941). «Studies on an agglutinogen (Rh) in human blood reacting with anti-rhesus sera and with human isoantibodies». The Journal of Experimental Medicine. 74 (4): 309–20. doi:10.1084/jem.74.4.309. PMC 2135190. PMID 19871137.
- ^ Levine P, Stetson RE (1939). «An unusual case of intragroup agglutination». JAMA. 113 (2): 126–7. doi:10.1001/jama.1939.72800270002007a.
- ^ Landsteiner K, Wiener AS (1940). «An agglutinable factor in human blood recognized by immune sera for rhesus blood». Proc Soc Exp Biol Med. 43: 223–4. doi:10.3181/00379727-43-11151. S2CID 58298368.
- ^ a b Levine P, Burnham L, Katzin E, Vogel P (December 1941). «The role of iso-immunization in the pathogenesis of erythroblastosis fetalis». American Journal of Obstetrics and Gynecology. 42 (6): 925–937. doi:10.1016/S0002-9378(41)90260-0. ISSN 0002-9378.
- ^ Avent ND, Reid ME (January 2000). «The Rh blood group system: a review». Blood. 95 (2): 375–87. doi:10.1182/blood.V95.2.375. PMID 10627438. S2CID 13803474.
- ^ Scott ML (July 2004). «The complexities of the Rh system». Vox Sanguinis. 87 (Suppl. 1): 58–62. doi:10.1111/j.1741-6892.2004.00431.x. PMID 15200606. S2CID 42103877.
External links[edit]
- Rh at BGMUT Blood Group Antigen Gene Mutation Database at NCBI, NIH
«Rh-» redirects here. For the Siddharta album, see Rh- (album). For the band, see The RH Factor. For the production company, see Regina Hall.
The Rh blood group system is a human blood group system. It contains proteins on the surface of red blood cells. After the ABO blood group system, it is the most likely to be involved in transfusion reactions. The Rh blood group system consists of 49 defined blood group antigens,[1] among which the five antigens D, C, c, E, and e are the most important. There is no d antigen. Rh(D) status of an individual is normally described with a positive (+) or negative (−) suffix after the ABO type (e.g., someone who is A+ has the A antigen and Rh(D) antigen, whereas someone who is A− has the A antigen but lacks the Rh(D) antigen). The terms Rh factor, Rh positive, and Rh negative refer to the Rh(D) antigen only. Antibodies to Rh antigens can be involved in hemolytic transfusion reactions and antibodies to the Rh(D) and Rh antigens confer significant risk of hemolytic disease of the fetus and newborn.
Nomenclature[edit]
| Fisher–Race | Wiener |
|---|---|
| Dce | R0 |
| DCe | R1 |
| DcE | R2 |
| DCE | RZ |
| dce | r |
| dCe | r’ |
| dcE | r″ |
| dCE | ry |
The Rh blood group system has two sets of nomenclatures: one developed by Ronald Fisher and R. R. Race, the other by Wiener. Both systems reflected alternative theories of inheritance. The Fisher–Race system, which is more commonly in use today, uses the CDE nomenclature. This system was based on the theory that a separate gene controls the product of each corresponding antigen (e.g., a «D gene» produces D antigen, and so on). However, the d gene was hypothetical, not actual.
The Wiener system used the Rh–Hr nomenclature. This system was based on the theory that there was one gene at a single locus on each of the 2 copies of chromosome 1, each contributing to production of multiple antigens. In this theory, a gene R1 is supposed to give rise to the «blood factors» Rh0, rh′, and rh″ (corresponding to modern nomenclature of the D, C, and E antigens) and the gene r to produce hr′ and hr″ (corresponding to modern nomenclature of the c and e antigens).[3]
Notations of the two theories are used interchangeably in blood banking (e.g., Rho(D) meaning RhD positive). Wiener’s notation is more complex and cumbersome for routine use. Because it is simpler to explain, the Fisher–Race theory has become more widely used.[citation needed]
DNA testing has shown that both are partially correct: There are in fact two linked genes, the RHD gene which produces a single immune specificity (anti-D) and the RHCE gene with multiple specificities (anti-C, anti-c, anti-E, anti-e). Thus, Wiener’s postulate that a gene could have multiple specificities (something many did not give credence to originally) has been proved to be correct. On the other hand, Wiener’s theory that there is only one gene has proved to be incorrect, as has the Fisher–Race theory that there are three genes, rather than the 2. The CDE notation used in the Fisher–Race nomenclature is sometimes rearranged to DCE to more accurately represent the co-location of the C and E encoding on the RhCE gene, and to make interpretation easier.[citation needed]
Antigens[edit]
The proteins which carry the Rh antigens are transmembrane proteins, whose structure suggest that they are ion channels.[4] The main antigens are D, C, E, c and e, which are encoded by two adjacent gene loci, the RHD gene which encodes the RhD protein with the D antigen (and variants)[5] and the RHCE gene which encodes the RhCE protein with the C, E, c and e antigens (and variants).[6] There is no d antigen. Lowercase «d» indicates the absence of the D antigen (the gene is usually deleted or otherwise nonfunctional).[citation needed]
1. This is the Rh-positive blood cell.
2. This is the Rh-negative blood cell.
3. These are the antigens on the Rh-positive blood cell that make it positive. The antigens allow the positive blood cell to attach to specific antibodies.
Rh phenotypes are readily identified through the presence or absence of the Rh surface antigens. As can be seen in the table below, most of the Rh phenotypes can be produced by several different Rh genotypes. The exact genotype of any individual can only be identified by DNA analysis. Regarding patient treatment, only the phenotype is usually of any clinical significance to ensure a patient is not exposed to an antigen they are likely to develop antibodies against. A probable genotype may be speculated on, based upon the statistical distributions of genotypes in the patient’s place of origin.[citation needed]
R0 (cDe or Dce) is today most common in Africa. The allele was thus often assumed in early blood group analyses to have been typical of populations on the continent; particularly in areas below the Sahara. Ottensooser et al. (1963) suggested that high R0 frequencies were likely characteristic of the ancient Judea Jews, who had emigrated from Egypt prior to their dispersal throughout the Mediterranean Basin and Europe[7] on the basis of high R0 percentages among Sephardi and Ashkenazi Jews compared to native European populations and the relative genetic isolation of Ashkenazim. However, more recent studies have found R0 frequencies as low as 24.3% among some Afroasiatic-speaking groups in the Horn of Africa,[8] as well as higher R0 frequencies among certain other Afroasiatic speakers in North Africa (37.3%)[9] and among some Palestinians in the Levant (30.4%).[10] On the contrary, at a frequency of 47.2% of the population of Basque country having the lack of the D antigen, these people display the highest frequency of the Rh negative phenotype.[11]
| Phenotype expressed on cell | Genotype expressed in DNA | Prevalence (%) |
|
|---|---|---|---|
| Fisher–Race notation | Wiener notation | ||
| D+ C+ E+ c+ e+ (RhD+) | Dce/DCE | R0RZ | 0.0125 |
| Dce/dCE | R0rY | 0.0003 | |
| DCe/DcE | R1R2 | 11.8648 | |
| DCe/dcE | R1r″ | 0.9992 | |
| DcE/dCe | R2r′ | 0.2775 | |
| DCE/dce | RZr | 0.1893 | |
| D+ C+ E+ c+ e− (RhD+) | DcE/DCE | R2RZ | 0.0687 |
| DcE/dCE | R2rY | 0.0014 | |
| DCE/dcE | RZr″ | 0.0058 | |
| D+ C+ E+ c− e+ (RhD+) | DCe/dCE | R1rY | 0.0042 |
| DCE/dCe | RZr′ | 0.0048 | |
| DCe/DCE | R1RZ | 0.2048 | |
| D+ C+ E+ c− e− (RhD+) | DCE/DCE | RZRZ | 0.0006 |
| DCE/dCE | RZrY | < 0.0001 | |
| D+ C+ E− c+ e+ (RhD+) | Dce/dCe | R0r′ | 0.0505 |
| DCe/dce | R1r | 32.6808 | |
| DCe/Dce | R1R0 | 2.1586 | |
| D+ C+ E− c− e+ (RhD+) | DCe/DCe | R1R1 | 17.6803 |
| DCe/dCe | R1r′ | 0.8270 | |
| D+ C− E+ c+ e+ (RhD+) | DcE/Dce | R2R0 | 0.7243 |
| Dce/dcE | R0r″ | 0.0610 | |
| DcE/dce | R2r | 10.9657 | |
| D+ C− E+ c+ e− (RhD+) | DcE/DcE | R2R2 | 1.9906 |
| DcE/dcE | R2r″ | 0.3353 | |
| D+ C− E− c+ e+ (RhD+) | Dce/Dce | R0R0 | 0.0659 |
| Dce/dce | R0r | 1.9950 | |
| D− C+ E+ c+ e+ (RhD−) | dce/dCE | rrY | 0.0039 |
| dCe/dcE | r′r″ | 0.0234 | |
| D− C+ E+ c+ e− (RhD−) | dcE/dCE | r″rY | 0.0001 |
| D− C+ E+ c− e+ (RhD−) | dCe/dCE | r′rY | 0.0001 |
| D− C+ E+ c− e− (RhD−) | dCE/dCE | rYrY | < 0.0001 |
| D− C+ E− c+ e+ (RhD−) | dce/dCe | rr′ | 0.7644 |
| D− C+ E− c− e+ (RhD−) | dCe/dCe | r′r′ | 0.0097 |
| D− C− E+ c+ e+ (RhD−) | dce/dcE | rr″ | 0.9235 |
| D− C− E+ c+ e− (RhD−) | dcE/dcE | r″r″ | 0.0141 |
| D− C− E− c+ e+ (RhD−) | dce/dce | rr | 15.1020 |
• Figures taken from a study performed in 1948 on a sample of 2000 people in the United Kingdom.[12]
| Rh Phenotype | CDE | Patients (%) | Donors (%) |
|---|---|---|---|
| R1r | CcDe | 37.4 | 33.0 |
| R1R2 | CcDEe | 35.7 | 30.5 |
| R1R1 | CDe | 5.7 | 21.8 |
| rr | ce | 10.3 | 11.6 |
| R2r | cDEe | 6.6 | 10.4 |
| R0R0 | cDe | 2.8 | 2.7 |
| R2R2 | cDE | 2.8 | 2.4 |
| rr″ | cEe | – | 0.98 |
| RZRZ | CDE | – | 0.03 |
| rr′ | Cce | 0.8 | – |
Rh antibodies[edit]
Rh antibodies are Immunoglobulin G (IgG) antibodies which are acquired through exposure to Rh-positive blood (generally either through pregnancy or transfusion of blood products). The D antigen is the most immunogenic of all the non-ABO antigens. Approximately 80% of individuals who are D-negative and exposed to a single D-positive unit will produce an anti-D antibody. The percentage of alloimmunization is significantly reduced in patients who are actively exsanguinating.[14]
All Rh antibodies except D display dosage (antibody reacts more strongly with red cells homozygous for an antigen than cells heterozygous for the antigen (EE stronger reaction vs Ee).
If anti-E is detected, the presence of anti-c should be strongly suspected (due to combined genetic inheritance). It is therefore common to select c-negative and E-negative blood for transfusion patients who have an anti-E and lack the c antigen (in general, a patient will not produce antibodies against their own antigens). Anti-c is a common cause of delayed hemolytic transfusion reactions.[15]
Hemolytic disease of the newborn[edit]
The hemolytic condition occurs when there is an incompatibility between the blood types of the mother and fetus. There is also potential incompatibility if the mother is Rh negative and the father is positive. When any incompatibility is detected, the mother often receives an injection at 28 weeks gestation and at birth to avoid the development of antibodies towards the fetus. These terms do not indicate which specific antigen-antibody incompatibility is implicated. The disorder in the fetus due to Rh D incompatibility is known as erythroblastosis fetalis.
- Hemolytic comes from two words: «hema» (blood) and «lysis» (solution) or breaking down of red blood cells
- Erythroblastosis refers to the making of immature red blood cells
- Fetalis refers to the fetus.
When the condition is caused by the Rh D antigen-antibody incompatibility, it is called Rh D Hemolytic disease of the newborn or Rh disease. Here, sensitization to Rh D antigens (usually by feto-maternal transfusion during pregnancy) may lead to the production of maternal IgG anti-D antibodies which can pass through the placenta. This is of particular importance to D negative females at or below childbearing age, because any subsequent pregnancy may be affected by the Rh D hemolytic disease of the newborn if the baby is D positive. The vast majority of Rh disease is preventable in modern antenatal care by injections of IgG anti-D antibodies (Rho(D) Immune Globulin). The incidence of Rh disease is mathematically related to the frequency of D negative individuals in a population, so Rh disease is rare in old-stock populations of Africa and the eastern half of Asia, and the Indigenous peoples of Oceania and the Americas, but more common in other genetic groups, most especially Western Europeans, but also other West Eurasians, and to a lesser degree, native Siberians, as well as those of mixed-race with a significant or dominant descent from those (e.g. the vast majority of Latin Americans and Central Asians).
- Symptoms and signs in the fetus:
- Enlarged liver, spleen, or heart and fluid buildup in the fetus’ abdomen seen via ultrasound.
- Symptoms and signs in the newborn:
- Anemia that creates the newborn’s pallor (pale appearance).
- Jaundice or yellow discoloration of the newborn’s skin, sclera or mucous membrane. This may be evident right after birth or after 24–48 hours after birth. This is caused by bilirubin (one of the end products of red blood cell destruction).
- Enlargement of the newborn’s liver and spleen.
- The newborn may have severe edema of the entire body.
- Dyspnea (difficulty breathing)
Other animals with Rh-like antigens causing hemolytic disease of the newborn
Rh disease only occurs in human fetuses however a similar disease called Neonatal isoerythrolysis (NI) can be observed in animal species of newborn horses, mules, pigs, cats, cattle, and dogs. What differs between Rh disease and NI is the pathogenesis of hemolysis between human fetuses and the animal species. With human mothers, the maternal antibodies are formed from the sensitization of foreign antigens of her unborn fetus’s red blood cells passing through the placenta causing hemolysis before birth, with other animals however, these maternal antibodies are not passed through the placenta but through colostrum. The newborn animal is without NI but soon develops hemolytic anemia after initial ingestion of its mother’s colostrum that contain antibodies that can be absorbed through the newborn’s intestines and are incompatible to its red blood cell antigen. After 48 hours of birth, the newborn may be allowed to nurse from its mother as her antibodies can no longer be absorbed through the neonate’s intestines. Because the most active newborn animals consume the most colostrum, they may be the ones who are most affected by the blood incompatibility of antigen and antibody.[16]
Rh proteins outside of human species
Rh molecules can be found in many different living organisms from worms, bacteria, algae, and other vertebrates. These Rh molecules from different animals have the same biochemical function-differing slightly in their amino acid sequences.[17] The Rh proteins in other species, however, do not correspond with the Rh blood group or antigens found on human red blood cells. One such example would be the nematode Caenorhabditis elegans. Because this worm does not have red blood cells, it cannot have Rh antigens, excluding it from having a Rh blood type. These Rh proteins therefore do not bind to red blood cells; they operate independently. Instead of transporting CO2 from the proteins of human red blood cells, C. elegan’s Rh proteins transport NH3 out of its body.[18]
Population data[edit]
According to a comprehensive study, the worldwide frequency of Rh-positive and Rh-negative blood types is approximately 94% and 6%, respectively. The same study concluded that the share of the population with Rh-negative blood type is set to fall further in the future primarily due to low population growth in Europe.[19] The frequency of Rh factor blood types and the RhD neg allele gene differs in various populations.[citation needed]
| Population | Rh(D) Neg | Rh(D) Pos | Rh(D) Neg alleles |
|---|---|---|---|
| African Americans | ∼ 7% | 93% | ∼ 26% |
| Albania[21] | 10.86% | 89% | weak D 1.4% |
| Basques[22] | 21%–36% | 65% | ∼ 60% |
| Britain[23] | 17% | 83% | |
| China[23] | < 1% | > 99% | |
| Ethiopians[23] | 1%–21% | 99%–79% | |
| Europeans (others) | 16% | 84% | 40% |
| India[23] | 0.6%–8.4% | 99.4%–91.6% | |
| Indonesia[23] | < 1% | > 99% | |
| Japan[23] | < 1% | > 99% | |
| Koreans[24] | < 1% | > 99% | |
| Madagascar[23] | 1% | 99% | |
| Moroccans[25] | 9.5% | 90.5% | |
| Moroccans (High Atlas)[26] | ∼ 29% | 71% | |
| Native Americans | ∼ 1% | 99% | ∼ 10% |
| Nigeria[27] | 6% | 94% | |
| Saudi Arabia[28] | 8.8% | 91.2% | 29.5% |
| Subequatorial Africa[23] | 1%–3% | 99%–97% | |
| United States[23] | 15% | 85% |
Genetics[edit]
This is a Punnett square for Rh factor inheritance. This square specifically shows two heterozygous Rh positive parents and the possible genotypes/phenotypes the offspring could have.
The D antigen is inherited as one gene (RHD) (on the short arm of the first chromosome, p36.13–p34.3) with various alleles. Typically, Rhesus positive people have an intact RHD gene while negative people lack the gene (or have mutations in it). However, there are exceptions: for instance, Japanese and black Africans may have an intact gene that is not expressed or only at very low levels.[29] The gene codes for the RhD protein on the red blood cell membrane. D− individuals who lack a functional RHD gene do not produce the D antigen, and may be immunized by D+ blood.[citation needed]
The D antigen is a dominant trait. If both of a child’s parents are Rh negative, the child will definitely be Rh negative. Otherwise the child may be Rh positive or Rh negative, depending on the parents’ specific genotypes.[30]
The epitopes for the next 4 most common Rh antigens, C, c, E and e are expressed on the highly similar RhCE protein that is genetically encoded in the RHCE gene, also found on chromosome 1. It has been shown that the RHD gene arose by duplication of the RHCE gene during primate evolution. Mice have just one RH gene.[31]
The RHAG gene, which is responsible for encoding Rh-associated glycoprotein (RhAG), is found on chromosome 6a.
The polypeptides produced from the RHD and RHCE genes form a complex on the red blood cell membrane with the Rh-associated glycoprotein.[15]
Function[edit]
| Blood group Rh C/E/D polypeptide | |
|---|---|
| Identifiers | |
| Symbol | ? |
| InterPro | IPR002229 |
On the basis of structural homology it has been proposed that the product of RHD gene, the RhD protein, is a membrane transport protein of uncertain specificity (CO2 or NH3) and unknown physiological role.[32][33] The three-dimensional structure of the related RHCG protein and biochemical analysis of the RhD protein complex indicates that the RhD protein is one of three subunits of an ammonia transporter.[34][35] Three recent studies[36][37][38] have reported a protective effect of the RhD-positive phenotype, especially RhD heterozygosity, against the negative effect of latent toxoplasmosis on psychomotor performance in infected subjects. RhD-negative compared to RhD-positive subjects without anamnestic titres of anti-Toxoplasma antibodies have shorter reaction times in tests of simple reaction times. And conversely, RhD-negative subjects with anamnestic titres (i.e. with latent toxoplasmosis) exhibited much longer reaction times than their RhD-positive counterparts. The published data suggested that only the protection of RhD-positive heterozygotes was long term in nature; the protection of RhD-positive homozygotes decreased with duration of the infection while the performance of RhD-negative homozygotes decreased immediately after the infection. The overall change in reaction times was always larger in the RhD-negative group than in the RhD-positive.[citation needed]
RHD polymorphism[edit]
Origin of RHD polymorphism[edit]
For a long time, the origin of RHD polymorphism was an evolutionary enigma.[39][40][41] Before the advent of modern medicine, the carriers of the rarer allele (e.g. RhD-negative women in a population of RhD positives or RhD-positive men in a population of RhD negatives) were at a disadvantage as some of their children (RhD-positive children born to preimmunised RhD-negative mothers) were at a higher risk of fetal or newborn death or health impairment from hemolytic disease.[citation needed]
Natural selection aside, the RHD-RHCE region is structurally predisposed to many mutations seen in humans, since the pair arose by gene duplication and remain similar enough for unequal crossing over to occur.[31] In addition to the case where D is deleted, crossover can also produce a single gene mixing exons from both RHD and RHCE, forming the majority of partial D types.[42]: 323
Weak D[edit]
| Weak D | Partial D | |
|---|---|---|
| Change in D | Decreased amount | Structural alternation |
| Can donate as if being: |
D positive | D positive |
| Can receive blood as if being: |
D positive (usually)[42] | D negative[15] |
In serologic testing, D positive blood is easily identified. Units that are D negative are often retested to rule out a weaker reaction. This was previously referred to as Du, which has been replaced.[42]: 322 By definition, weak D phenotype is characterized by negative reaction with anti-D reagent at immediate spin (IS), negative reaction after 37 °C incubation, and positive reaction at anti-human globulin (AHG) phase. Weak D phenotype can occur in several ways. In some cases, this phenotype occurs because of an altered surface protein that is more common in people of European descent. An inheritable form also occurs, as a result of a weakened form of the R0 gene. Weak D may also occur as «C in trans», whereby a C gene is present on the opposite chromosome to a D gene (as in the combination R0r’, or «Dce/dCe»). The testing is difficult, since using different anti-D reagents, especially the older polyclonal reagents, may give different results.
The practical implication of this is that people with this sub-phenotype will have a product labeled as «D positive» when donating blood. When receiving blood, they are sometimes typed as a «D negative», though this is the subject of some debate. Most «Weak D» patients can receive «D positive» blood without complications.[42]: 323 However, it is important to correctly identify the ones that have to be considered D+ or D−. This is important, since most blood banks have a limited supply of «D negative» blood and the correct transfusion is clinically relevant. In this respect, genotyping of blood groups has much simplified this detection of the various variants in the Rh blood group system.
Partial D[edit]
It is important to differentiate weak D (due to a quantitative difference in the D antigen) from partial D (due to a qualitative difference in the D antigen). Simply put, the weak D phenotype is due to a reduced number of D antigens on a red blood cell. In contrast, the partial D phenotype is due to an alteration in D-epitopes. Thus, in partial D, the number of D antigens is not reduced but the protein structure is altered. These individuals, if alloimmunized to D, can produce an anti-D antibody. Therefore, partial D patients who are donating blood should be labeled as D-positive but, if receiving blood, they should be labeled as D-negative and receive D-negative units.[15]
In the past, partial D was called ‘D mosaic’ or ‘D variant.’ Different partial D phenotypes are defined by different D epitopes on the outer surface of the red blood cell membrane. More than 30 different partial D phenotypes have been described.[15]
Rhnull phenotype[edit]
Rhnull individuals have no Rh antigens (no Rh or RhAG) on their red blood cells.[43] This rare condition[43] has been called «Golden Blood».[44] As a consequence of Rh antigen absence, Rhnull red blood cells also lack LW and Fy5 and show weak expression of S, s, and U antigens.
Red blood cells lacking Rh/RhAG proteins have structural abnormalities (such as stomatocytosis) and cell membrane defects that can result in hemolytic anemia.[15][43]
The first Rhnull blood was discovered in an Aboriginal Australian woman, in 1961.[45] Only 43 individuals have been reported to have it worldwide. Only nine active donors have been reported.[44] Its properties make it attractive in numerous medical applications, but scarcity makes it expensive to transport and acquire.[46]
Other Rh group antigens[edit]
Currently, 50 antigens have been described in the Rh group system; among those described here, the D, C, c, E and e antigens are the most important. The others are much less frequently encountered or are rarely clinically significant. Each is given a number, though the highest assigned number (CEVF or RH61 according to the ISBT terminology) is not an accurate reflection of the antigens encountered since many (e.g. Rh38) have been combined, reassigned to other groups, or otherwise removed.[42]: 324
Some of the other Rh «antigens» are f («ce», RH6), Ce (RH7), Cw (RH8), Cx (RH9), V (RH10), Ew (RH11), G (RH12), Tar (RH40), VS (RH20), Dw (RH23), and CE (RH22). Some of these groups, including f, Ce and CE, describe grouping of some existing groups. Others, like V, describe an epitope created by some other mutation on the RHD and RHCE genes. V in particular is caused by a mutation on RHCE.[47]
History[edit]
The term «Rh» was originally an abbreviation of «Rhesus factor». It was discovered in 1937 by Karl Landsteiner and Alexander S. Wiener, who, at the time, believed it to be a similar antigen found in rhesus macaque red blood cells. It was subsequently discovered that the human factor is not identical to the rhesus monkey factor, but by then, «Rhesus Group» and like terms were already in widespread, worldwide use. Thus, notwithstanding it is a misnomer, the term survives (e.g., rhesus blood group system and the obsolete terms rhesus factor, rhesus positive, and rhesus negative – all three of which actually refer specifically and only to the Rh D factor and are thus misleading when unmodified). Contemporary practice is to use «Rh» as a term of art instead of «Rhesus» (e.g., «Rh Group», «Rh factors», «Rh D», etc.).
The significance of their discovery was not immediately apparent and was only realized in 1940, after subsequent findings by Philip Levine and Rufus Stetson.[48] The serum that led to the discovery was produced by immunizing rabbits with red blood cells from a rhesus macaque. The antigen that induced this immunization was designated by them as Rh factor to indicate that rhesus blood had been used for the production of the serum.[49]
In 1939, Phillip Levine and Rufus Stetson published in a first case report the clinical consequences of non-recognized Rh factor, hemolytic transfusion reaction, and hemolytic disease of the newborn in its most severe form.[50] It was recognized that the serum of the reported woman agglutinated with red blood cells of about 80% of the people although the then known blood groups, in particular ABO were matched. No name was given to this agglutinin when described. In 1940, Karl Landsteiner and Alexander S. Wiener made the connection to their earlier discovery, reporting a serum that also reacted with about 85% of different human red blood cells.[51]
In 1941, Group O: a patient of Dr. Paul in Irvington, NJ, delivered a normal infant in 1931; this pregnancy was followed by a long period of sterility. The second pregnancy (April, 1941) resulted in an infant with icterus gravis.[52] In May 1941, the third anti-Rh serum (M.S.) of Group O became available.[52]
Based on the serologic similarities, Rh factor was later also used for antigens, and anti-Rh for antibodies, found in humans such as those previously described by Levine and Stetson. Although differences between these two sera were shown already in 1942 and clearly demonstrated in 1963, the already widely used term «Rh» was kept for the clinically described human antibodies which are different from the ones related to the rhesus monkey. This real factor found in rhesus macaque was classified in the Landsteiner-Weiner antigen system (antigen LW, antibody anti-LW) in honor of the discoverers.[53][54]
It was recognized that the Rh factor was just one in a system of various antigens. Based on different models of genetic inheritance, two different terminologies were developed; both of them are still in use.
The clinical significance of this highly immunizing D antigen (i.e., Rh factor) was soon realized. Some keystones were to recognize its importance for blood transfusion (including reliable diagnostic tests), hemolytic disease of the newborn (including exchange transfusion), and very importantly the prevention of it by screening and prophylaxis.
The discovery of fetal cell-free DNA in maternal circulation by Holzgrieve et al. led to the noninvasive genotyping of fetal Rh genes in many countries.
References[edit]
- ^ Dean, Laura. Blood Groups and Red Cell Antigens [Internet].. Bethesda (MD): National Center for Biotechnology Information (US); 2005, Chapter. 7.
- ^ «Rh System». Canadian Blood Services at learnserology.ca. Retrieved 2021-01-19.
- ^ Weiner AS (1 February 1949). «Genetics and Nomenclature of the Rh–Hr Blood Types». Antonie van Leeuwenhoek. 15 (1): 17–28. doi:10.1007/BF02062626. ISSN 0003-6072. S2CID 35680084.
- ^ «dbRBC – Blood Group Antigen Gene Mutation Database». www.ncbi.nlm.nih.gov. Archived from the original on 2011-02-13. Retrieved 2010-06-15.
- ^ «RHD Rh blood group, D antigen [Homo sapiens] – Gene Result». nlm.nih.gov. Retrieved 2010-06-15.
- ^ «RHCE Rh blood group, CcEe antigens [Homo sapiens] – Gene Result». nlm.nih.gov. Archived from the original on 2010-03-20. Retrieved 2010-06-15.
- ^ Ottensooser F, Leon N, Sato M, Saldanha PH (March 1963). «Blood groups of a population of Ashkenazi Jews in Brazil». American Journal of Physical Anthropology. 21 (1): 41–8. doi:10.1002/ajpa.1330210106. PMID 13940710. S2CID 21795826.
- ^ Harrison GA, Küchemann CF, Moore MA, Boyce AJ, Baju T, Mourant AE, et al. (1969). «The effects of altitudinal variation in Ethiopian populations». Philosophical Transactions of the Royal Society of London B: Biological Sciences. 256 (805): 147–182. Bibcode:1969RSPTB.256..147H. doi:10.1098/rstb.1969.0040.
- ^ Metri AA, Sidi-Yakhlef A, Biémont C, Saidi M, Chaif O, Ouraghi SA (2012). «A genetic study of nine populations from the region of Tlemcen in Western Algeria: a comparative analysis on the Mediterranean scale». Anthropological Science. 120 (3): 209–216. doi:10.1537/ase.120618. Archived from the original on 29 August 2017. Retrieved 28 August 2017.
- ^ El-Wahhab Skaik YA (July 2011). «The Rh allele frequencies in Gaza city in Palestine». Asian Journal of Transfusion Science. 5 (2): 150–2. doi:10.4103/0973-6247.83241. PMC 3159245. PMID 21897594.
- ^ Flores-Bello A, Mas-Ponte D, Rosu ME, Bosch E, Calafell F, Comas D (December 2018). «Sequence diversity of the Rh blood group system in Basques». European Journal of Human Genetics. 26 (12): 1859–1866. doi:10.1038/s41431-018-0232-1. PMC 6244411. PMID 30089826.
- ^ Race RR, Mourant AE (June 1948). «The Rh chromosome frequencies in England». Blood. 3 (6): 689–95. doi:10.1182/blood.V3.6.689.689. PMID 18860341.
- ^ Canatan D, Acar N, Kiliç B (1999). «Rh Subgroups and Kell Antigens in Patients With Thalassemia and in Donors in Turkey» (PDF). Turkish Journal of Medical Sciences. 29: 155–7. Archived from the original (PDF) on 2008-12-17. Retrieved 2008-10-17.
- ^ Roback et al. AABB Technical Manual, 16th Ed. Bethesda, AABB Press, 2008.
- ^ a b c d e f Mais, DD. ASCP Quick Compendium of Clinical Pathology, 2nd Ed. Chicago, ASCP Press, 2009.
- ^ Cotter S (June 1, 2001). Hematology. Quick Look Series (1st ed.). Teton NewMedia. pp. 32–33. ISBN 978-1893441361.
- ^ Ji Q, Hashmi S, Liu Z, Zhang J, Chen Y, Huang CH (April 2006). «CeRh1 (rhr-1) is a dominant Rhesus gene essential for embryonic development and hypodermal function in Caenorhabditis elegans». Proceedings of the National Academy of Sciences of the United States of America. 103 (15): 5881–5886. doi:10.1073/pnas.0600901103. PMC 1458667. PMID 16595629.
- ^ «What is Rhesus factor? Did we get it from Rhesus monkeys? Can Rh factor be found in other animals?». The Tech Interactive. 2018-11-20. Retrieved 2022-10-24.
- ^ «Blood Type Frequencies by Country including the Rh Factor – Rhesus Negative».
- ^ Mack S (March 21, 2001). «Re: Is the RH negative blood type more prevalent in certain ethnic groups?». MadSci Network. Archived from the original on February 24, 2011.
- ^ Xhetani M, Seferi I, Férec C, Zoraqi G, Fichou Y (October 2014). «Distribution of Rhesus blood group antigens and weak D alleles in the population of Albania». Blood Transfusion = Trasfusione del Sangue. 12 (4): 565–9. doi:10.2450/2014.0240-13. PMC 4212038. PMID 24960662.
- ^ Touinssi M, Chiaroni J, Degioanni A, De Micco P, Dutour O, Bauduer F (2004). «Distribution of rhesus blood group system in the French basques: a reappraisal using the allele-specific primers PCR method». Human Heredity. 58 (2): 69–72. doi:10.1159/000083027. PMID 15711086. S2CID 44542508.
- ^ a b c d e f g h i Golassa L, Tsegaye A, Erko B, Mamo H (July 2017). «High rhesus (Rh(D)) negative frequency and ethnic-group based ABO blood group distribution in Ethiopia». BMC Research Notes. 10 (1): 330. doi:10.1186/s13104-017-2644-3. PMC 5530478. PMID 28747227.
- ^ Kim JY, Kim SY, Kim CA, Yon GS, Park SS (March 2005). «Molecular characterization of D- Korean persons: development of a diagnostic strategy». Transfusion. 45 (3): 345–52. doi:10.1111/j.1537-2995.2005.04311.x. PMID 15752151. S2CID 42899178.
- ^ Wafi ME, Housse HE, Nourichafi N, Bouisk K, Benajiba M, Habti N (2016). «Prevalence of weak D phenotype among D negative C/E+ blood donors in Morocco» (PDF). International Journal of Blood Transfusion and Immunohematology. 6 (1): 3–7. doi:10.5348/ijbti-2016-22-OA-2. Archived (PDF) from the original on August 28, 2016. Retrieved February 3, 2018.
- ^ Weinstock C (January 2014). «It is worthwhile filling in the remaining blank spots for blood group antigen frequencies». Blood Transfusion = Trasfusione del Sangue. 12 (1): 3–6. doi:10.2450/2013.0192-13. PMC 3926725. PMID 24120599.
- ^ Enosolease ME, Bazuaye GN (January 2008). «Distribution of ABO and Rh-D blood groups in the Benin area of Niger-Delta: Implication for regional blood transfusion». Asian Journal of Transfusion Science. 2 (1): 3–5. doi:10.4103/0973-6247.39502. PMC 2798756. PMID 20041069.
- ^ Eweidah MH, Rahiman S, Ali MH, Al-Shamary AM (April 2011). «Distribution of ABO and Rhesus (RHD) Blood Groups in Al-Jouf Province of the Saudi Arabia» (PDF). The Anthropologist. 13 (2): 99–102. doi:10.1080/09720073.2011.11891182. S2CID 75537061. Archived (PDF) from the original on January 2, 2013. Retrieved February 3, 2018.
- ^ Avent ND, Reid ME (January 2000). «The Rh blood group system: a review». Blood. 95 (2): 375–387. doi:10.1182/blood.V95.2.375. PMID 10627438. S2CID 13803474.
- ^ «ABO inheritance patterns». Inheritance patterns of blood groups. Australian Red Cross Blood Service. Archived from the original on 1 November 2013. Retrieved 30 October 2013.
- ^ a b Wagner FF, Flegel WA (March 2002). «RHCE represents the ancestral RH position, while RHD is the duplicated gene». Blood. 99 (6): 2272–3. doi:10.1182/blood-2001-12-0153. PMID 11902138.
- ^ Kustu S, Inwood W (2006). «Biological gas channels for NH3 and CO2: evidence that Rh (Rhesus) proteins are CO2 channels». Transfusion Clinique et Biologique. 13 (1–2): 103–10. doi:10.1016/j.tracli.2006.03.001. PMID 16563833.
- ^ Biver S, Scohy S, Szpirer J, Szpirer C, André B, Marini AM (2006). «Physiological role of the putative ammonium transporter RhCG in the mouse». Transfusion Clinique et Biologique. 13 (1–2): 167–8. doi:10.1016/j.tracli.2006.03.003. PMID 16564721.
- ^ Gruswitz F, Chaudhary S, Ho JD, Schlessinger A, Pezeshki B, Ho CM, et al. (May 2010). «Function of human Rh based on structure of RhCG at 2.1 A». Proceedings of the National Academy of Sciences of the United States of America. 107 (21): 9638–43. Bibcode:2010PNAS..107.9638G. doi:10.1073/pnas.1003587107. PMC 2906887. PMID 20457942.
- ^ Westhoff CM (January 2007). «The structure and function of the Rh antigen complex». Seminars in Hematology. 44 (1): 42–50. doi:10.1053/j.seminhematol.2006.09.010. PMC 1831834. PMID 17198846.
- ^ Novotná M, Havlícek J, Smith AP, Kolbeková P, Skallová A, Klose J, et al. (September 2008). «Toxoplasma and reaction time: role of toxoplasmosis in the origin, preservation and geographical distribution of Rh blood group polymorphism». Parasitology. 135 (11): 1253–61. doi:10.1017/S003118200800485X. PMID 18752708. S2CID 5956901.
- ^ Flegr J, Novotná M, Lindová J, Havlícek J (August 2008). «Neurophysiological effect of the Rh factor. Protective role of the RhD molecule against Toxoplasma-induced impairment of reaction times in women» (PDF). Neuro Endocrinology Letters. 29 (4): 475–81. PMID 18766148.
- ^ Flegr J, Klose J, Novotná M, Berenreitterová M, Havlícek J (May 2009). «Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study». BMC Infectious Diseases. 9: 72. doi:10.1186/1471-2334-9-72. PMC 2692860. PMID 19470165.
- ^ Haldane JB (1942). «Selection against heterozygosis in Man». Annals of Eugenics. 11: 333–340. doi:10.1111/j.1469-1809.1941.tb02297.x.
- ^ Fisher RA, Race RR, Taylor GL (1944). «Mutation and the Rhesus reaction». Nature. 153 (3873): 106. Bibcode:1944Natur.153..106F. doi:10.1038/153106b0. S2CID 2104065.
- ^ Li CC (1953). «Is the Rh facing a crossroad? A critique of the compensation effect». Am Nat. 87 (835): 257–261. doi:10.1086/281782. S2CID 84715943.
- ^ a b c d e Brecher ME (2005). Technical Manual (15th ed.). Bethesda MD: American Association of Blood Banks. ISBN 978-1-56395-196-1.
- ^ a b c Cartron JP (December 1999). «RH blood group system and molecular basis of Rh-deficiency». Bailliere’s Best Practice & Research. Clinical Haematology. 12 (4): 655–89. doi:10.1053/beha.1999.0047. PMID 10895258.
- ^ a b «Rhnull, the Rarest Blood Type on Earth, Has Been Called the «Golden Blood»«. Curiosity.com. Archived from the original on 5 December 2019. Retrieved 2019-06-05.
- ^ «J-STAGE».
- ^ Bailey P. «The man with the golden blood». Mosaic Science. Retrieved 16 January 2019.
- ^ «Rh System: Anti-V». Professional Education. 2 October 2019. Archived from the original on 7 November 2020.
- ^ Landsteiner K, Wiener AS (1940). «An Agglutinable Factor in Human Blood Recognized by Immune Sera for Rhesus Blood». Exp Biol Med (Maywood). 43 (1): 223. doi:10.3181/00379727-43-11151. S2CID 58298368.
- ^ Landsteiner K, Wiener AS (September 1941). «Studies on an agglutinogen (Rh) in human blood reacting with anti-rhesus sera and with human isoantibodies». The Journal of Experimental Medicine. 74 (4): 309–20. doi:10.1084/jem.74.4.309. PMC 2135190. PMID 19871137.
- ^ Levine P, Stetson RE (1939). «An unusual case of intragroup agglutination». JAMA. 113 (2): 126–7. doi:10.1001/jama.1939.72800270002007a.
- ^ Landsteiner K, Wiener AS (1940). «An agglutinable factor in human blood recognized by immune sera for rhesus blood». Proc Soc Exp Biol Med. 43: 223–4. doi:10.3181/00379727-43-11151. S2CID 58298368.
- ^ a b Levine P, Burnham L, Katzin E, Vogel P (December 1941). «The role of iso-immunization in the pathogenesis of erythroblastosis fetalis». American Journal of Obstetrics and Gynecology. 42 (6): 925–937. doi:10.1016/S0002-9378(41)90260-0. ISSN 0002-9378.
- ^ Avent ND, Reid ME (January 2000). «The Rh blood group system: a review». Blood. 95 (2): 375–87. doi:10.1182/blood.V95.2.375. PMID 10627438. S2CID 13803474.
- ^ Scott ML (July 2004). «The complexities of the Rh system». Vox Sanguinis. 87 (Suppl. 1): 58–62. doi:10.1111/j.1741-6892.2004.00431.x. PMID 15200606. S2CID 42103877.
External links[edit]
- Rh at BGMUT Blood Group Antigen Gene Mutation Database at NCBI, NIH
Группы крови
При переливании необходимо предварительно определить группу крови и донора и пациента.
Существует 4 группы крови.
Система ABO
В конце XIX в. Австралийский ученый Карл Ландштайнер, проводя исследование эритроцитов, обнаружил любопытную закономерность: в красных кровяных клетках (эритроцитах) некоторых людей может быть специальный маркер, который ученый обозначил буквой А, у других — маркер В, у третьих не обнаруживались ни А, ни В. Позже выяснилось, что описанные Ландштайнером маркеры — особые белки, определяющие видовую специфичность клеток, или антигены. Фактически эти исследования поделили все человечество на 3 группы крови.
Четвертая группа была описана в 1902 году учеными Декастелло и Штурли. Совместное открытие ученых получило название системы АВО.
О (I)
первая группа крови
А (II)
вторая группа крови
В (III)
третья группа крови
АВ (IV)
четвертая группа крови
Резус-фактор
В отличие от антигенов группы крови, резус-фактор-это антиген, обнаруженный только в мембране эритроцита и не зависящий от других факторов крови. Резус-фактор передается по наследству и сохраняется в течение всей жизни человека. 85% людей, в эритроцитах которых находится резус-фактор, обладают резус-положительной кровью (Rh+), кровь остальных людей не содержит резус-фактор и называется резус-отрицательной (Rh-).
Келл-фактор
Система Kell — это система группы крови, в которую входят 25 антигенов, в том числе самый иммуногенный после А, В и D, антиген К.
На основании наличия антигена K в эритроцитах или его отсутствия все люди могут быть разделены на две группы: Kell-отрицательные и Kell-положительные. Наличие антигена К (Kell-положительный) не является патологией и передается по наследству, как и другие групповые антигены человека. В России он встречается у 7-10% жителей.
В настоящее время в учреждениях службы крови определяют наличие антигена К, как наиболее опасного для возникновения иммунологических осложнений. Описаны многие случаи гемотрансфузионных осложнений и гемолитической болезни новорожденных, причиной которых была изоиммунизация антигеном К.
Kell-отрицательным должна переливаться только кровь от доноров, не имеющих антиген К для предотвращения гемолиза. Лица же Kell-положительные являются универсальными реципиентами крови, так как у них не происходит отторжения её компонентов.
В целях профилактики посттрансфузионных осложнений, обусловленных антигеном К системы Kell, отделения и станции переливания крови выдают для переливания в лечебные учреждения эритроцитную взвесь или массу, не содержащие этого фактора. При переливании всех видов плазмы, тромбоцитного концентрата, лейкоцитного концентрата антиген К системы Kell не учитывают.
Поэтому Kell-положительным донорам рекомендуется донорство плазмы.
Читайте также
Современная эффективная деятельность Службы крови стала возможна только благодаря реализации основных направлений ее развития.
Большинство людей знают о донорстве очень мало и потому доверяют самым необоснованным мифам…
Успешные практики организации донорских дней, мнения ведущих экспертов Службы крови
Скачивайте первое в России приложение для доноров

Заместитель генерального директора по науке и образованию
Ведущий врач
Кандидат медицинских наук
Гематолог
Определение резус-фактора наряду с определением группы крови является базовым анализом, позволяющим определять совместимость людей по иммунологическим характеристикам крови. Совместимость по резус-фактору имеет значение при переливании крови и зачатии.
Что такое резус-фактор
К середине XX-го века ученые получили подтверждение, что совместимость людей по иммунологическим характеристикам крови не исчерпывается классическим разделением на четыре группы крови. Был обнаружен белок, присутствующий в крови примерно 85% людей. Соответственно, приблизительно у 15% людей он отсутствует. При переливании крови от первых ко вторым, организм реципиента начинает вырабатывать антитела к этому белку, то есть это вещество является антигеном. В медицине данный белок обозначается латинской буквой D. Если белок D в Вашей крови, то значит, у Вас резус-фактор положительный (Rh+), и Вы принадлежите к большинству (85%). Если данного антигена в Вашей крови нет, Ваш резус-фактор отрицательный (Rh-). Резус-фактор в течение жизни не изменяется.
Почему резус-фактор важен при переливании крови
При переливании крови от человека с Rh+ к человеку с Rh- происходит конфликт по резус-фактору. Но если при конфликте по группе крови разрушение эритроцитов (гемолиз) начинается сразу, то при конфликте по резус-фактору при первом переливании гемолиза не бывает. Первый контакт с антигеном D приводит лишь к сенсибилизации реципиента, то есть его организм вырабатывает специфические антитела, чувствительность к антигену повышается. А вот если антиген попадает в кровь человека с отрицательным резус-фактором повторно, организм начинает реагировать на проникновение чужеродного агента: эритроциты начинают слипаться внутри сосудов и разрушаться. Человек чувствует стеснение в груди, затрудненность дыхания, боли в области поясницы. Понижается артериальное давление, развивается острая почечная недостаточность. Подобный комплекс симптомов называется гемолитическим шоком.
В настоящее время гемолитический шок при переливании крови практически исключен. В сегодняшней медицинской практике для переливания используется кровь, совпадающая по группе и по резус-фактору с кровью реципиента. Для того чтобы исключить ошибку, перед проведением любой операции анализ на определение группы крови и резус-фактора делается заново.
Резус-фактор при планировании беременности
Резус-фактор также имеет значение при планировании беременности. Если у мужчины резус-фактор положительный, а у женщины – отрицательный, то у их ребенка в большинстве случаев окажется положительный резус-фактор. То есть возникает ситуация, когда у матери Rh-, а у плода – Rh+. В этом случае существует риск конфликта по резус-фактору при беременности. Такая ситуация требует особого внимания при ведении беременности. Необходимо будет сдавать анализы на определения титра и класса антирезусных антител в крови.
В норме кровь матери и кровь ребенка в процессе вынашивания не смешиваются, так как существует плацентарный барьер. Поэтому антиген D в кровь матери не попадает, и антитела к нему не вырабатываются. Поэтому первая беременность с конфликтом по резус-фактору, как правило, протекает без осложнений. Однако во время родов половые пути травмируются, при этом могут пострадать и кожные покровы ребенка, а значит, велика вероятность попадания антигена в кровь матери. Происходит сенсибилизация организма матери, и теперь следующая беременность может быть осложнена. Особенно велик риск сенсибилизации при родах с помощью кесарева сечения. Если первая беременность была искусственно прервана (аборт) или закончилась выкидышем, то риск сенсибилизации матери также возрастает.
В некоторых случаях – при сахарном диабете, гестозе, гриппе или ОРВИ, перенесенных во время беременности – защита плода оказывается ослабленной, и процесс выработки антител начинается еще в период вынашивания.
Если антитела в крови матери уже присутствуют, при новой беременности они могут проникнуть сквозь плацентарный барьер и губительно сказаться на состоянии плода (гемолитическая болезнь плода). Под воздействием антител эритроциты ребенка начинают разрушаться, что приводит к поражениям печени, почек, головного мозга. Возрастает вероятность выкидыша.
Своевременное лечение при резус-конфликте позволяет значительно снизить риск развития наиболее тяжелых осложнений гемолитической болезни.
Риск возникновения конфликта по резус-фактору не является противопоказанием для беременности и не может быть основанием для её прерывания.
Как сдать анализ на определение резус-фактора
Анализ на определение резус-фактора обычно сдается вместе с анализом на определение группы крови. Кровь, как правило, берется из вены (в некоторых случаях могут взять кровь из пальца). Специальной подготовки к анализу не требуется. Однако нежелательно сдавать анализ сразу после приема пищи (с момента последней трапезы должно пройти не менее 4-х часов).
Где сдать анализ на определение резус-фактора в Москве
Анализ на определение резус-фактора Вы можете сдать в любой из поликлиник «Семейного доктора».
Есть вопросы?
Оставьте телефон –
и мы Вам перезвоним
Записаться на диагностику
Не занимайтесь самолечением. Обратитесь к нашим специалистам, которые правильно поставят диагноз и назначат лечение.
Определение группы крови и резус-фактора
Фото: Shutterstock/FOTODOM
- Врачи
- Статья обновлена: 18 июня 2020
Группа крови (АВ0), Резус-фактор (Rh-фактор) – лабораторные исследования, которые позволяют определить группу крови по системе АВ0 и оценить резус-принадлежность человека.
Какие бывают группы крови?
Различия между людьми по группам крови — это различия по составу определенных антигенов и антител. Основная система классификации крови — система AB0 (читается — а, б, ноль). Группы кpoви обозначают по наличию или отсутствию определенного типа «склеивающего» фактора (агглютиногена):
0 (I) — 1-я группа крови
А (II) — 2-я группа крови
В (III) — 3-я группа крови
АВ (IV) — 4-я группа крови
Что такое резус-фактор?
Резус-фактор представляет собой антиген (белок), который находится в эритроцитах. Наличие резус-фактора не зависит от групповой принадлежности по системе АВ0, не изменяется в течение жизни и не зависит от внешних факторов. Примерно 80-85% людей имеют его и соответственно являются резус-положительными. Те же, у кого его нет – резус-отрицательными.
Какие существуют показания к назначению анализа на группу крови и резус-фактор?
- определение совместимости для переливания крови;
- гемолитическая болезнь новорожденных (выявление несовместимости крови матери и плода по системе АВ 0);
- предоперационная подготовка;
- беременность (подготовка и наблюдение в динамике беременных с отрицательным резус-фактором).
Анализ готовится 1 день.
В какой форме выдается результат анализа на группу крови и резус фактор?
- 0 (I) — первая группа,
- A (II) — вторая группа,
- B (III) — третья группа,
- AB (IV) — четвертая группа крови.
Rh + (положительная) или Rh – (отрицательная).
Источники
- Navabi J., Navabi SM., Hemmati N., Shaahmadi Z., Aghaei A. Higher Odds of Type 2 Diabetes for Some Blood Groups. // Public Health Genomics — 2020 — Vol23 — N1-2 — p.37-41; PMID:32252060
- Hejna M., Birner P., Preusser M., Thallinger CM., Worel N., Asari R., Dolak W., Schmid R., Schoppmann SF., Raderer M. Lack of correlation between blood group and HER-2 status in adenocarcinomas of the upper gastrointestinal tract. // Mol Clin Oncol — 2013 — Vol1 — N6 — p.1079-1083; PMID:24649296
- Duplantie J., Gonzales OM., Bois A., Nshimyumukiza L., Gekas J., Bujold E., Morin V., Vallée M., Giguère Y., Gagné C., Rousseau F., Reinharz D. Cost-effectiveness of the management of rh-negative pregnant women. // J Obstet Gynaecol Can — 2013 — Vol35 — N8 — p.730-740; PMID:24007709